978-0134663890 Chapter 2 Part 2
2-14$
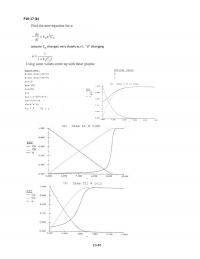
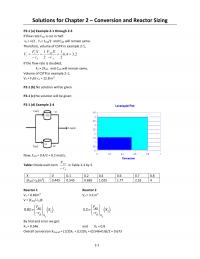
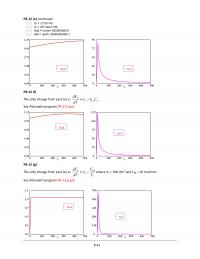
P2-10)(e)$
The$amount$of$catalyst$necessary$to$achieve$40$%$conversion$in$a$single$PBR$can$be$found$from$
calculating$the$area$of$the$shaded$region$in$the$graph$below.$
$
The$necessary$catalyst$weight$is$approximately$13$kg.$$
The$necessary$catalyst$weight$is$approximately$13$kg.$$
The$necessary$catalyst$weight$is$approximately$13$kg.$
$
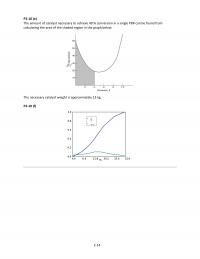
P2-10)(f)$
$
$
$
$
)
$
3-1$
Solutions)for)Chapter)3))Rate)Laws)
)
P3-1)(a))$
(i)$Individualized$solution$
(ii)$2550$K$
(iii)$Individualized$solution$
)
P3-1)(b)$
(i)$The$equilibrium$concentration$changes,$but$the$equilibrium$conversion$remains$the$same$in$all$the$
three$cases$(50%).$The$time$taken$to$attain$equilibrium$remains$the$same$in$all$the$three$cases.$
$
(ii)$The$trajectories$remain$similar,$but$the$time$taken$to$attain$equilibrium$changes,$as$the$rate$constants$are$lower.$$(iii)$The$forward/reverse$reaction$goes$to$completion.$$(iv)$This$is$the$nature$of$a$stochastic$simulation.$The$number$of$molecules$in$the$simulation$are$very$less,$as$compared$to$the$large$number$in$the$deterministic$model$(number$of$molecules$are$of$the$order$of$the$Avogadro$number).$The$fluctuations$reduce$as$you$increase$the$number$of$molecules,$and$the$stochastic$model$is$identical$to$a$deterministic$model$when$there$are$infinite$molecules.$$(v)$The$fluctuations$in$the$trajectories$reduce.$$$(vi)$No,$equilibrium$only$means$that$the$forward$reaction$rate$is$equal$to$the$reverse$reaction$rate.$It$does$not$mean$that$they$stop$occurring.$This$can$be$seen$from$the$fact$that$there$is$always$a$small$fluctuation$about$the$equilibrium$concentration.$$
(ii)$The$trajectories$remain$similar,$but$the$time$taken$to$attain$equilibrium$changes,$as$the$rate$constants$are$lower.$$(iii)$The$forward/reverse$reaction$goes$to$completion.$$(iv)$This$is$the$nature$of$a$stochastic$simulation.$The$number$of$molecules$in$the$simulation$are$very$less,$as$compared$to$the$large$number$in$the$deterministic$model$(number$of$molecules$are$of$the$order$of$the$Avogadro$number).$The$fluctuations$reduce$as$you$increase$the$number$of$molecules,$and$the$stochastic$model$is$identical$to$a$deterministic$model$when$there$are$infinite$molecules.$$(v)$The$fluctuations$in$the$trajectories$reduce.$$$(vi)$No,$equilibrium$only$means$that$the$forward$reaction$rate$is$equal$to$the$reverse$reaction$rate.$It$does$not$mean$that$they$stop$occurring.$This$can$be$seen$from$the$fact$that$there$is$always$a$small$fluctuation$about$the$equilibrium$concentration.$$
(ii)$The$trajectories$remain$similar,$but$the$time$taken$to$attain$equilibrium$changes,$as$the$rate$
constants$are$lower.$
$
(iii)$The$forward/reverse$reaction$goes$to$completion.$
$
(iv)$This$is$the$nature$of$a$stochastic$simulation.$The$number$of$molecules$in$the$simulation$are$very$less,$
as$compared$to$the$large$number$in$the$deterministic$model$(number$of$molecules$are$of$the$order$of$
the$Avogadro$number).$The$fluctuations$reduce$as$you$increase$the$number$of$molecules,$and$the$
stochastic$model$is$identical$to$a$deterministic$model$when$there$are$infinite$molecules.$
$
(v)$The$fluctuations$in$the$trajectories$reduce.$$
$
(vi)$No,$equilibrium$only$means$that$the$forward$reaction$rate$is$equal$to$the$reverse$reaction$rate.$It$
does$not$mean$that$they$stop$occurring.$This$can$be$seen$from$the$fact$that$there$is$always$a$small$
fluctuation$about$the$equilibrium$concentration.$$
$
P3-1)(c)$
$
3-2$
P3-1)(c))Continued$
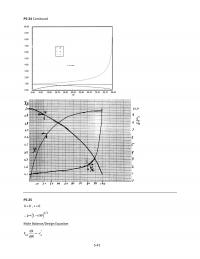
ln =ln
1
$
From$the$graph$of$ln$k$vs$1/T$above,$we$get:$
ln =27.577,
=10889 $
Thus,$
=9.474 10!! !!, =90.531 /$
=9.474 10!! exp 10889 !!$
=9.474 10!! exp 10889 !!$
=9.474 10!! exp
10889
!!$
$
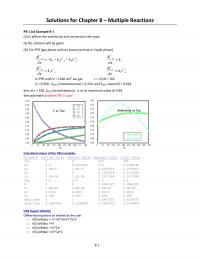
P3-1)(d))Example)3-1$
For,$E$=$60kJ/mol$ $ $ $ $$$$$$$$$$$$For,$E$=$240kJ/mol$ $
$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$ $
$
T)(K)$
k)(1/sec)$
1/T$
ln(k)$
$
T)(K)$
k)(1/sec)$
1/T$
ln(k)$
310$
1023100$
0.003226$
13.83918$
$
310$
4.78E-25$
0.003226$
-56.0003$
315$
1480488$
0.003175$
14.2087$
$
315$
2.1E-24$
0.003175$
-54.5222$
320$
2117757$
0.003125$
14.56667$
$
320$
8.77E-24$
0.003125$
-53.0903$
325$
2996152$
0.003077$
14.91363$
$
325$
3.51E-23$
0.003077$
-51.7025$
330$
4194548$
0.00303$
15.25008$
$
330$
1.35E-22$
0.00303$
-50.3567$
335$
5813595$
0.002985$
15.57648$
$
335$
4.98E-22$
0.002985$
-49.0511$
$
$
$
3-3$
P3-1)(e))No$solution$will$be$given$
P3-1)(f)$
A+1
2
B1
2
C
$
Rate$law:$ $$and$
$
$
$ $ $
==12.5$
==12.5$
==12.5
$
$
)
P3-2)(a)$
Refer$to$Fig$3-4$
The$fraction$of$molecular$collisions$having$energies$less$than$or$equal$to$35$Kcal$is$given$by$the$area$
under$the$curve,$f(E,T)dE$$from$EA$=$0$to$35$Kcal.$
under$the$curve,$f(E,T)dE$$from$EA$=$0$to$35$Kcal.$
under$the$curve,$f(E,T)dE$$from$EA$=$0$to$35$Kcal.$
$
P3-2(b))$
The$fraction$of$molecular$collisions$having$energies$between$10$and$20$Kcal$is$given$by$the$area$under$
the$curve$f(E,T)$from$EA$=$10$to$20$Kcal.$
the$curve$f(E,T)$from$EA$=$10$to$20$Kcal.$
the$curve$f(E,T)$from$EA$=$10$to$20$Kcal.$
$
P3-2)(c)$
The$fraction$of$molecular$collisions$having$energies$greater$than$the$activation$energy$EA=$25$Kcal$is$
given$by$the$area$under$the$curve$f(E,T)$from$$EA$=25$to$50$Kcal.$
given$by$the$area$under$the$curve$f(E,T)$from$$EA$=25$to$50$Kcal.$
given$by$the$area$under$the$curve$f(E,T)$from$$EA$=25$to$50$Kcal.$
$
)
P3-3)(a)$
$
$
(a)$
(b)$
$
)
P3-4)No$solution$will$be$given$
$
3-4$
P3-5)(a)$
The$fraction$of$collisions$having$energy$between$E$=$3$and$E$=$5$is$the$area$under$the$graph$between$
those$two$boundaries$=$2*0.5*(0.1875$+$0.25)$=$0.4375$
those$two$boundaries$=$2*0.5*(0.1875$+$0.25)$=$0.4375$
those$two$boundaries$=$2*0.5*(0.1875$+$0.25)$=$0.4375$
P3-5)(b)$
$
P3-5)(c)$
$
P3-5)(d)$
Since$f(E,T)$=$0$for$E$>$8$kcal,$the$fraction$with$energies$greater$than$8$kcal$=$0$
$
$
$
)
P3-6)(a)$$
Note:$This$problem$can$have$many$solutions$as$data$fitting$can$be$done$in$many$ways.$
Using$Arrhenius$Equation$
For$Fire$flies:$
T(in$K)$
1/T$
Flashes/min$
ln(flashes/min)$
294$
0.003401$
9$
2.197$
298$
0.003356$
12.16$
2.498$
303$
0.003300$
16.2$
2.785$
$
Plotting$ln$(flashes/min)$vs.$1/T,$$
We$get$a$straight$line.$
$
For$Crickets:$$
T(in$K)$
1/T$x103$
chirps/min$
ln(chirps/min)$
287.2$
3.482$
80$
4.382$
293.3$
3.409$
126$
4.836$
300$
3.333$
200$
5.298$
$
Plotting$ln$(chirps/min)$Vs$1/T,$$
We$get$a$straight$line.$
Both,$Fireflies$and$Crickets$data$$
follow$the$Arrhenius$Model.$ln$y$$=$A$+$B/T$,$and$have$the$similar$activation$energy.$
follow$the$Arrhenius$Model.$ln$y$$=$A$+$B/T$,$and$have$the$similar$activation$energy.$
follow$the$Arrhenius$Model.$
ln$y$$=$A$+$B/T$,$and$have$the$similar$activation$energy.$
)
)
)
)
3-5$
P3-6)(b)$$
For$Honeybee:$$
T(in$K)$
1/T$x103$
V(cm/s)$
ln(V)$
298$
3.356$
0.7$
-0.357$
303$
3.300$
1.8$
0.588$
308$
3.247$
3$
1.098$
$
Plotting$ln$(V)$vs.$1/T,$almost$straight$line.$
ln$(V)$=$44.6$$1.33E4/T$$At$T$=$40oC$(313K)$V$=$6.4cm/s$At$T$=$-5oC$(268K)$V$=$0.005cm/s$(But$bee$would$not$be$alive$at$this$temperature)$
ln$(V)$=$44.6$$1.33E4/T$$At$T$=$40oC$(313K)$V$=$6.4cm/s$At$T$=$-5oC$(268K)$V$=$0.005cm/s$(But$bee$would$not$be$alive$at$this$temperature)$
ln$(V)$=$44.6$$1.33E4/T$
$
At$T$=$40oC$(313K)$V$=$6.4cm/s$
At$T$=$-5oC$(268K)$V$=$0.005cm/s$(But$bee$would$
not$be$alive$at$this$temperature)$
$
P3-6)(c)$$
For$Ants:$$
T(in$K)$
1/T$x103$
V(cm/s)$
ln(V)$
283$
3.53$
0.5$
-0.69$
293$
3.41$
2$
0.69$
303$
3.30$
3.4$
1.22$
311$
3.21$
6.5$
1.87$
$
Plotting$ln$(V)$$vs.$$1/T,$$
We$get$almost$a$straight$line.$$
$
So$activity$of$bees,$ants,$crickets$and$fireflies$follow$Arrhenius$model.$So$activity$increases$with$an$
increase$in$temperature.$Activation$energies$for$fireflies$and$crickets$are$almost$the$same.$
$
Insect$
Activation$Energy$
Cricket$
52150$
Firefly$
54800$
Ant$
95570$
Honeybee$
141800$
$
P3-6)(d)$$
There$is$a$limit$to$temperature$for$which$data$for$any$one$of$the$insect$can$be$extrapolate.$Data$which$
would$be$helpful$is$the$maximum$and$the$minimum$temperature$that$these$insects$can$endure$before$
death.$Therefore,$even$if$extrapolation$gives$us$a$value$that$looks$reasonable,$at$certain$temperature$it$could$be$useless.$
death.$Therefore,$even$if$extrapolation$gives$us$a$value$that$looks$reasonable,$at$certain$temperature$it$could$be$useless.$
death.$Therefore,$even$if$extrapolation$gives$us$a$value$that$looks$reasonable,$at$certain$temperature$it$
could$be$useless.$
$
P3-6)(e)$
1)$The$rate$at$which$the$beetle$can$push$a$ball$of$dung$is$directly$proportional$to$its$rate$constant,$
therefore$
-rA$=c*k,$where$c$is$a$constant$related$to$the$mass$of$the$beetle$and$the$dung$and$k$is$the$rate$constant$
=!!!!" $
=!!!!" $
=
!!!
!" $
$
3-6$
P3-6)(e))Continued$
From$the$data$given$
$$$$$$$$$-rA$
$$$$$$T(K)$
$
1/T$
ln$k$
6.5$
300$
$
0.003333$
1.871802$
13$
310$
$
0.003226$
2.564949$
18$
313$
$
0.003195$
2.890372$
$
Refer$to$P3-8$(similar$procedure)$
Therefore,$A$=$1.299X1011$$$
$$$$$$$$$$$$$$$$$$$$$E$=$59195.68$J/mol$k$=$1.299X1011$exp(-7120/T)$Now$at$T$=$41.5$C$=$314.5$K$k$=$19.12$cm/s$Therefore,$beetle$can$push$dung$at$19.12$cm/s$at$41.5$C$
$$$$$$$$$$$$$$$$$$$$$E$=$59195.68$J/mol$k$=$1.299X1011$exp(-7120/T)$Now$at$T$=$41.5$C$=$314.5$K$k$=$19.12$cm/s$Therefore,$beetle$can$push$dung$at$19.12$cm/s$at$41.5$C$
$$$$$$$$$$$$$$$$$$$$$E$=$59195.68$J/mol$
k$=$1.299X1011$exp(-7120/T)$
Now$at$T$=$41.5$C$=$314.5$K$
k$=$19.12$cm/s$
Therefore,$beetle$can$push$dung$at$19.12$cm/s$at$41.5$C$
$
P3-6)(e))$
2))Individualized$solution$
$
)
P3-7$
There$are$two$competing$effects$that$bring$about$the$maximum$in$the$corrosion$rate:$Temperature$and$
HCN-H2SO4$concentration.$The$corrosion$rate$increases$with$increasing$temperature$and$increasing$
concentration$of$HCN-H2SO4$complex.$The$temperature$increases$as$we$go$from$top$to$bottom$of$the$column$and$consequently$the$rate$of$corrosion$should$increase.$However,$the$HCN$concentrations$(and$the$HCN-H2SO4$complex)$decrease$as$we$go$from$top$to$bottom$of$the$column.$There$is$virtually$no$HCN$in$the$bottom$of$the$column.$These$two$opposing$factors$results$in$the$maximum$of$the$corrosion$rate$somewhere$around$the$middle$of$the$column.$
concentration$of$HCN-H2SO4$complex.$The$temperature$increases$as$we$go$from$top$to$bottom$of$the$column$and$consequently$the$rate$of$corrosion$should$increase.$However,$the$HCN$concentrations$(and$the$HCN-H2SO4$complex)$decrease$as$we$go$from$top$to$bottom$of$the$column.$There$is$virtually$no$HCN$in$the$bottom$of$the$column.$These$two$opposing$factors$results$in$the$maximum$of$the$corrosion$rate$somewhere$around$the$middle$of$the$column.$
concentration$of$HCN-H2SO4$complex.$The$temperature$increases$as$we$go$from$top$to$bottom$of$the$
column$and$consequently$the$rate$of$corrosion$should$increase.$However,$the$HCN$concentrations$(and$
the$HCN-H2SO4$complex)$decrease$as$we$go$from$top$to$bottom$of$the$column.$There$is$virtually$no$
HCN$in$the$bottom$of$the$column.$These$two$opposing$factors$results$in$the$maximum$of$the$corrosion$
rate$somewhere$around$the$middle$of$the$column.$
$
)
y$=$-7120.4x$+$25.593$
R$=$0.9893$
0)
0.5)
1)
1.5)
2)
2.5)
3)
3.5)
0.00318) 0.00321) 0.00324) 0.00327) 0.0033) 0.00333) 0.00336)
ln(k))
1/T))(K-1)$
3-7$Since, = !"!"$$$therefore$ln$A$=$41.99$and$E/R$=$14999$
3-7$Since, = !"!"$$$therefore$ln$A$=$41.99$and$E/R$=$14999$
3-7$
Since, = !"
!"$$$therefore$ln$A$=$41.99$and$E/R$=$14999$
P3-8)Antidote$did$not$dissolve$from$glass$at$low$temperatures.$
$
)
P3-9)(a)$
If$a$reaction$rate$doubles$for$an$increase$in$10C,$at$T$=$T1$let$k$=$k1$and$at$T$=$T2$=$T1+10,$let$k$=$k2$=$2k1.$$
Then$with$k$=$Ae-E/RT$in$general,$ $and$ $,$or$
$$$
$$$or$$$$
$
$
Therefore:$
$
$
$
$
which$can$be$approximated$by$ .$Consequently,$for$this$doubling$rate$fule$of$thumb$to$
be$valid,$the$temperature$at$which$the$doubling$will$take$place$must$be$related$to$the$activation$energy$
by$this$relationship.$$
by$this$relationship.$$
by$this$relationship.$$
$
P3-9)(b))Individualized$solution$
$
)
P3-10)$
From$the$$given$data$
-rA(dm3/mol.s)$
T(K)$
k=$-rA/(4*1.5)$
1/T$(!!)$
ln$(k)$
0.002$
300$
0.00033333$
0.003333$
-8.00637$
0.046$
320$
0.00766667$
0.003125$
-4.87087$
0.72$
340$
0.12$
0.002941$
-2.12026$
8.33$
360$
1.38833333$
0.002778$
0.328104$
Plotting$ln(k)$vs$(1/T),$we$have$a$straight$line:$$
$
3-8$
P3-10)(a)$
Activation)energy)(E),))
$$
$E$=$14999*8.314$=124700$J/mol$=$124.7)kJ/mol$)
$E$=$14999*8.314$=124700$J/mol$=$124.7)kJ/mol$)
$E$=$14999*8.314$=124700$J/mol$=$124.7)kJ/mol$
)
P3-10)(b)$
Frequency)Factor)(A),)ln)A)=)41.99)))))$
$=1.72 10!" !"!
!"#!.!
!)$
)
)
)
P3-10)(c)$
$=1.72
Essentials of Chemical Reaction Engineering 2nd Edition