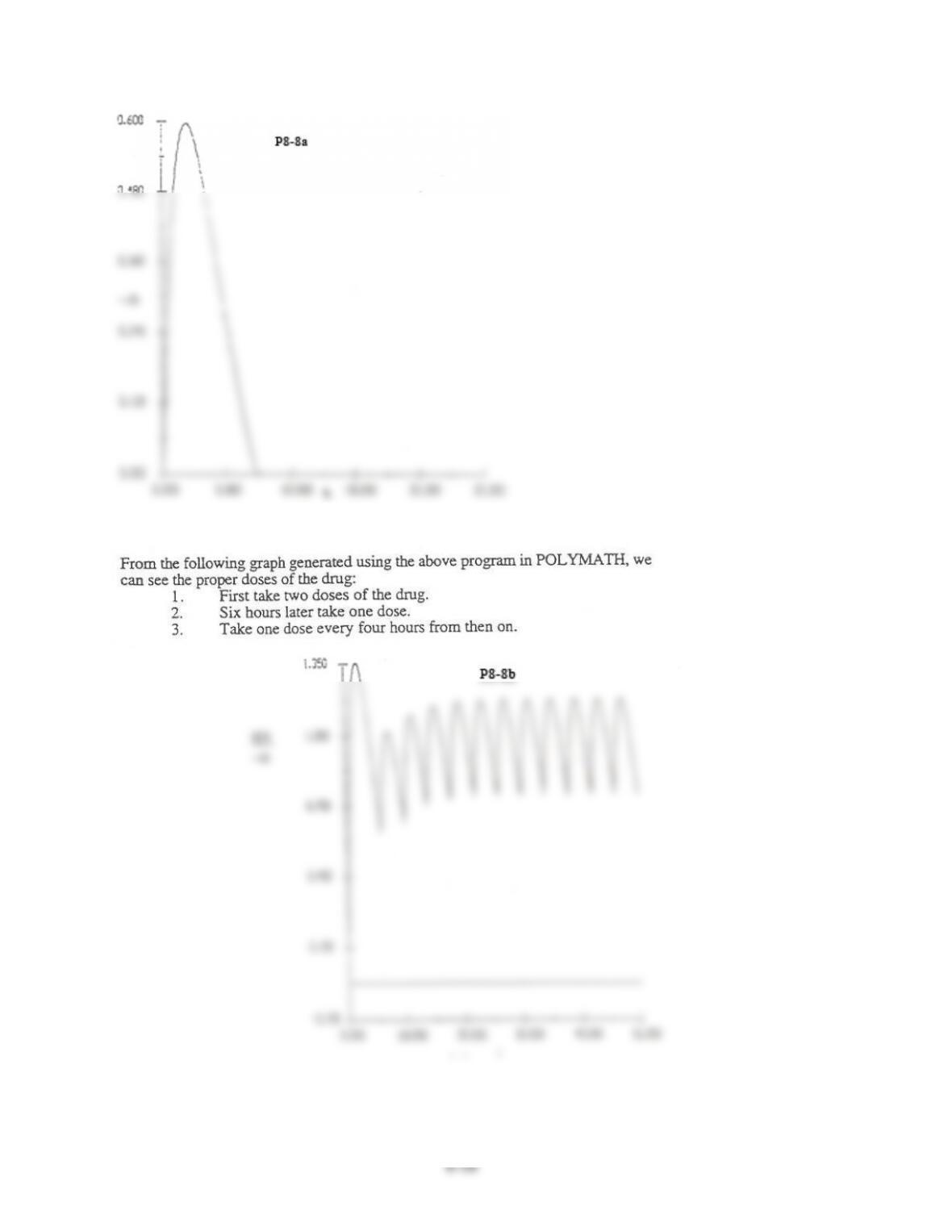
8-8$
P8-3)(a)))
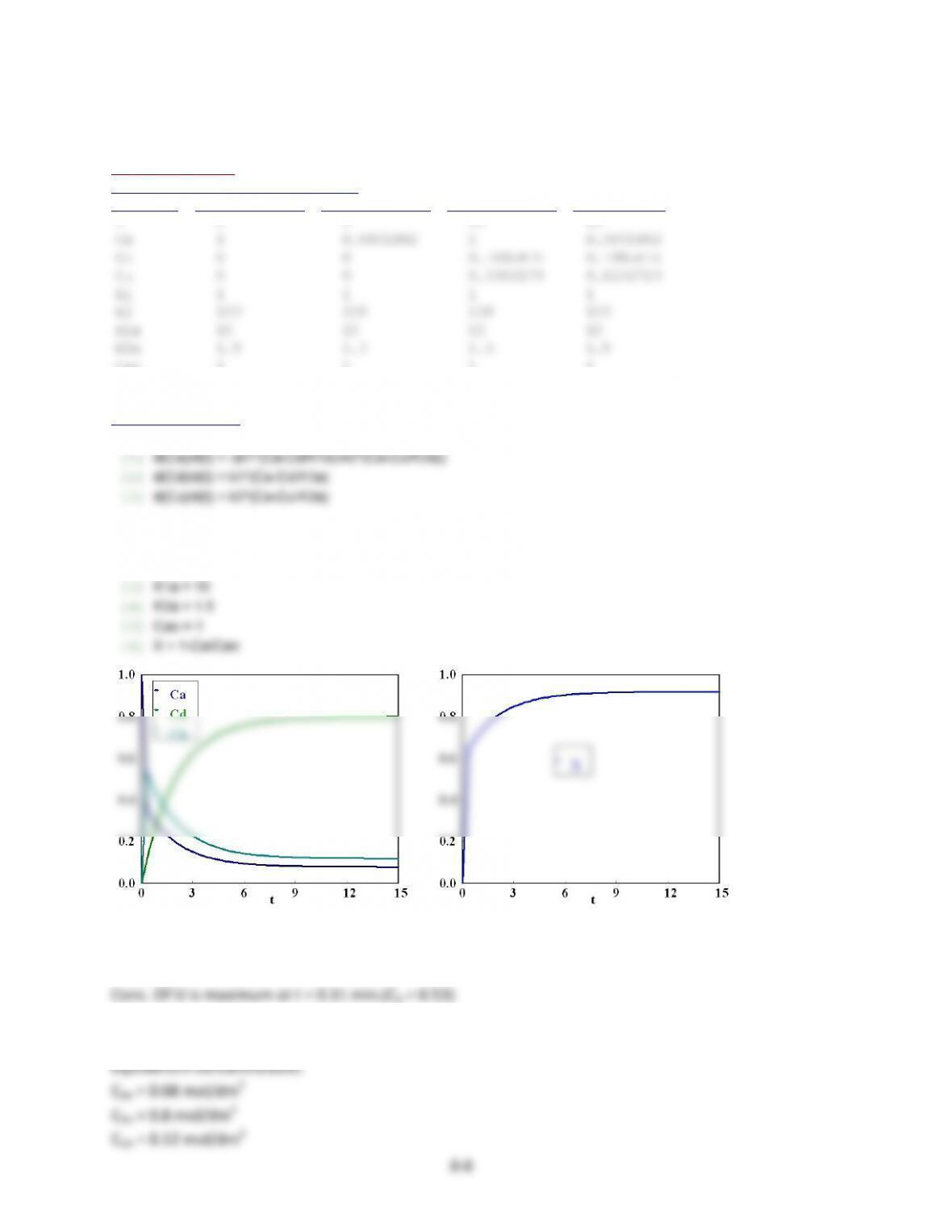
Plot$of$CA,$CD$and$CU$as$a$function$of$time$(t):$$
See$Polymath$program$P8-3-a.pol.$
POLYMATH)Results
Calculated)values)of)the)DEQ)variables
Variable initial value minimal value maximal value final value
t 0 0 15 15
Ca 1 0.0801802 1 0.0801802
X 0 0 0.9198198 0.9198198
ODE)Report)(RKF45)
Differential equations as entered by the user
[1] d(Ca)/d(t) = -(k1*(Ca-Cd/K1a)-k2*(Ca-Cu/K2a))
Explicit equations as entered by the user
[1] k1 = 1.0
[2] k2 = 100
[3] K1a = 10
To$maximize$CD$stop$the$reaction$after$a$long$time.$The$concentration$of$D$only$increases$with$time$
)
P8-3)(b)))
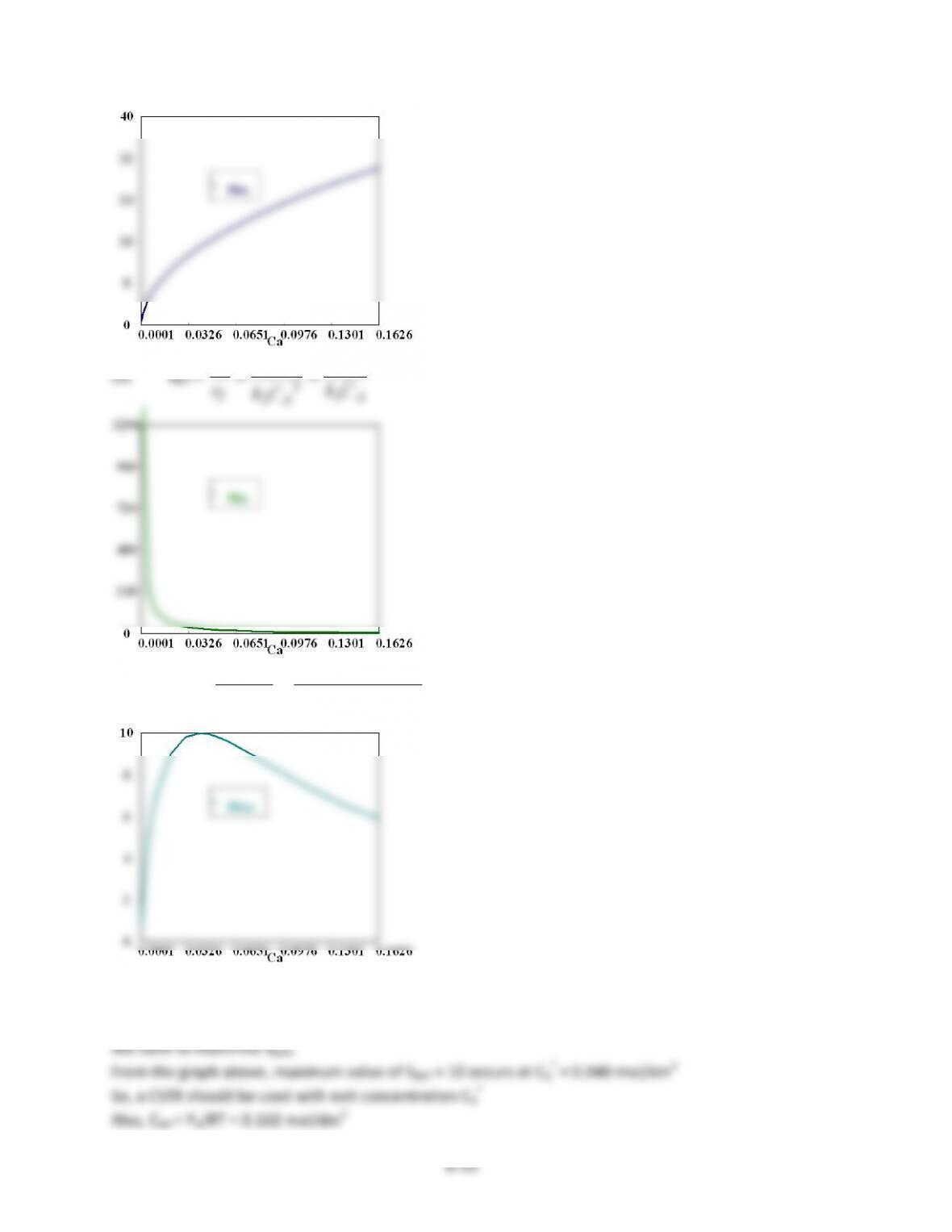
Conc.$Of$U$is$maximum$at$t$=$0.31$min.(CA$=$0.53)$
)
P8-3)(c))
Equilibrium$concentrations:$