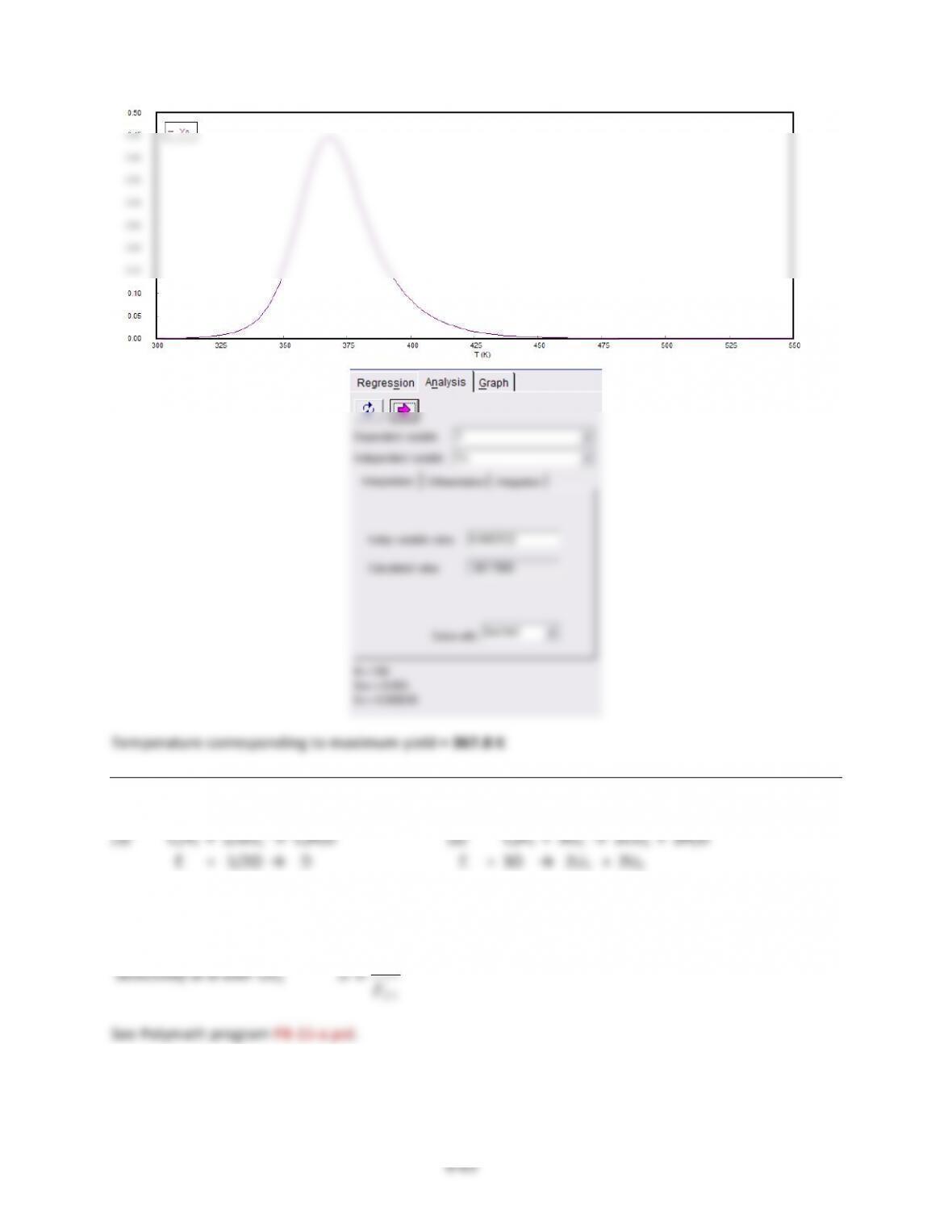
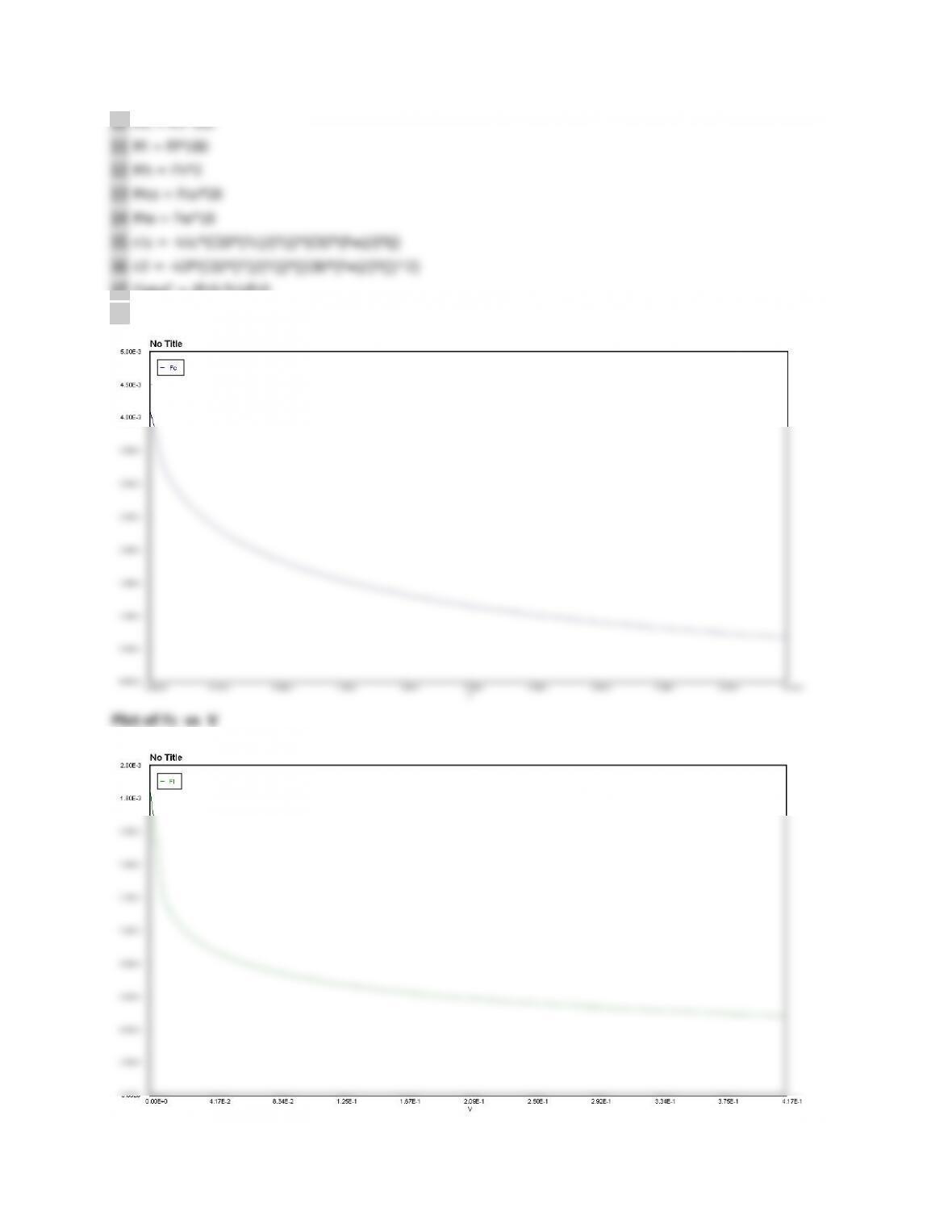
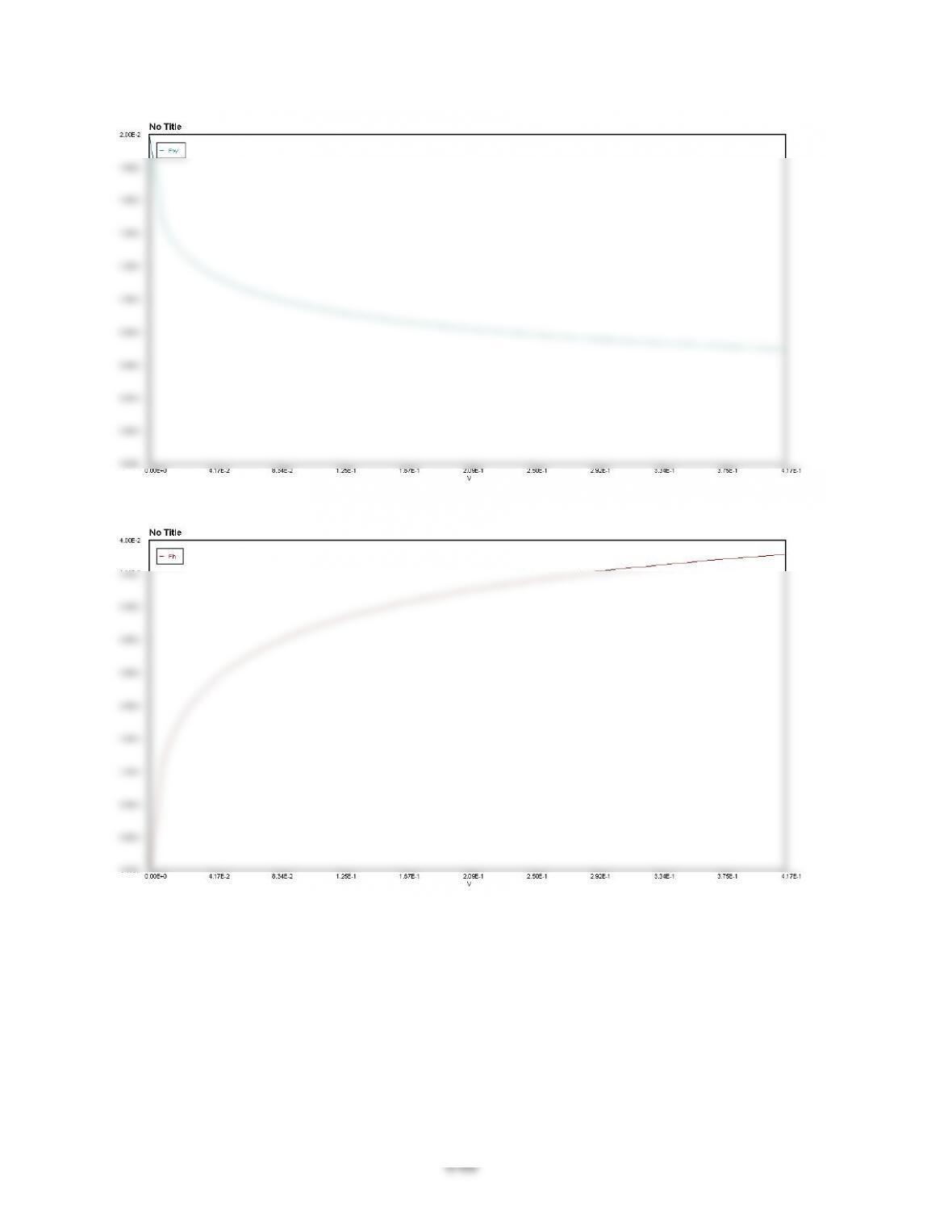
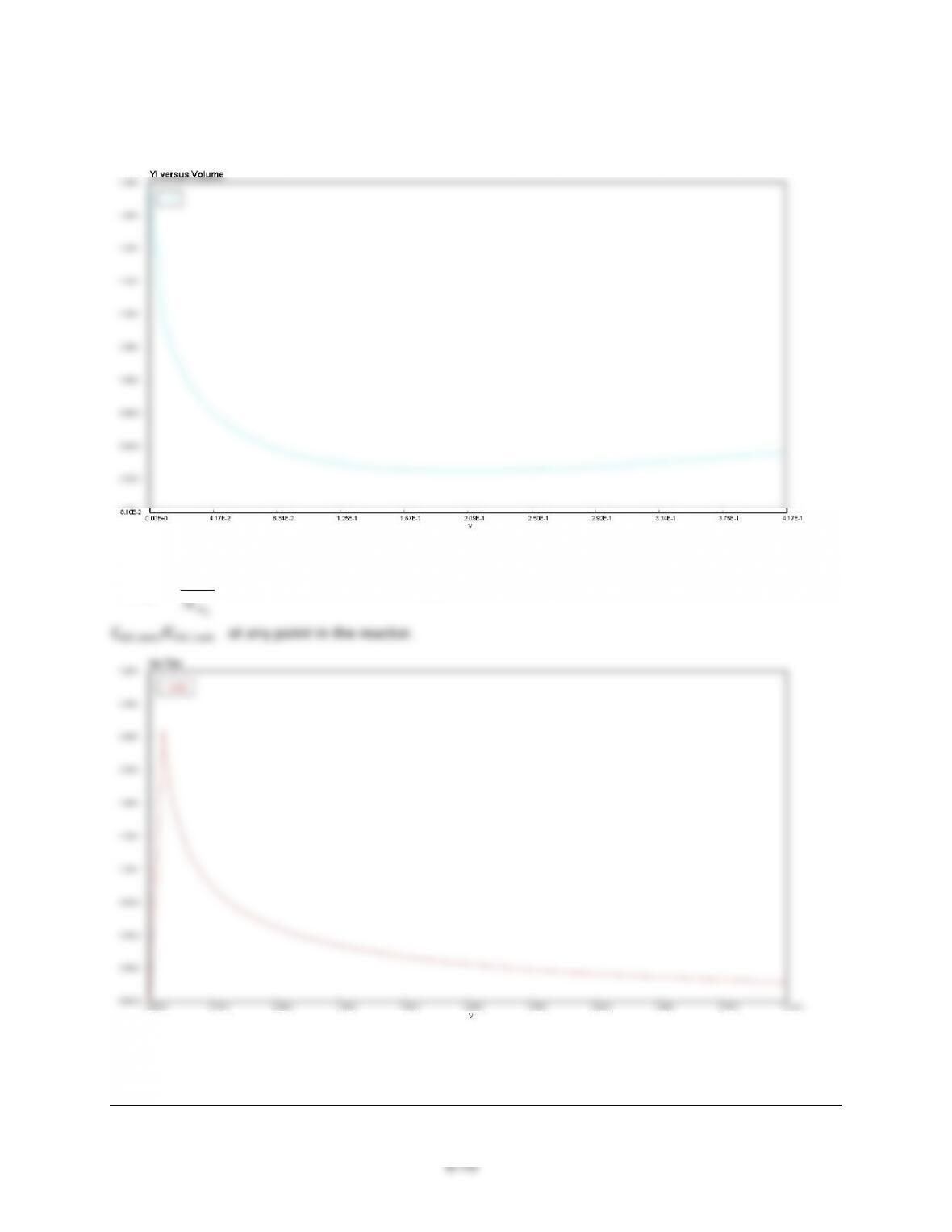
9-4$
P9-1)(e))Example)9-5)Continued$
$$$ $
$
Notice$that$uncompetitive$inhibition$by$the$substrate$causes$the$concentration$of$cells$to$decrease$with$
time,$ whereas$ without$ uncompetitive$ inhibition,$ the$ concentration$ of$ cells$ increased$ with$ time.$$
Consequently,$ the$ concentration$ of$ product$ is$ very$ low$ compared$ to$ the$ case$ without$ uncompetitive$
inhibition.$
$
(vii)$
Change$the$observed$reaction$rate$constant:$
$
$
All$other$equations$are$the$same$as$in$part$(v).$
Since$CP$is$so$small,$the$factor$
$
whether$CP
*$=$93$g/dm3$or$CP
*$=$10,000$g/dm3.$$Thus$the$
plots$for$this$part$are$approximately$the$same$as$the$plots$in$part$(v).$
$
P9-1)(f))Example)on)the)Professional)Reference)Shelf)R9.1$
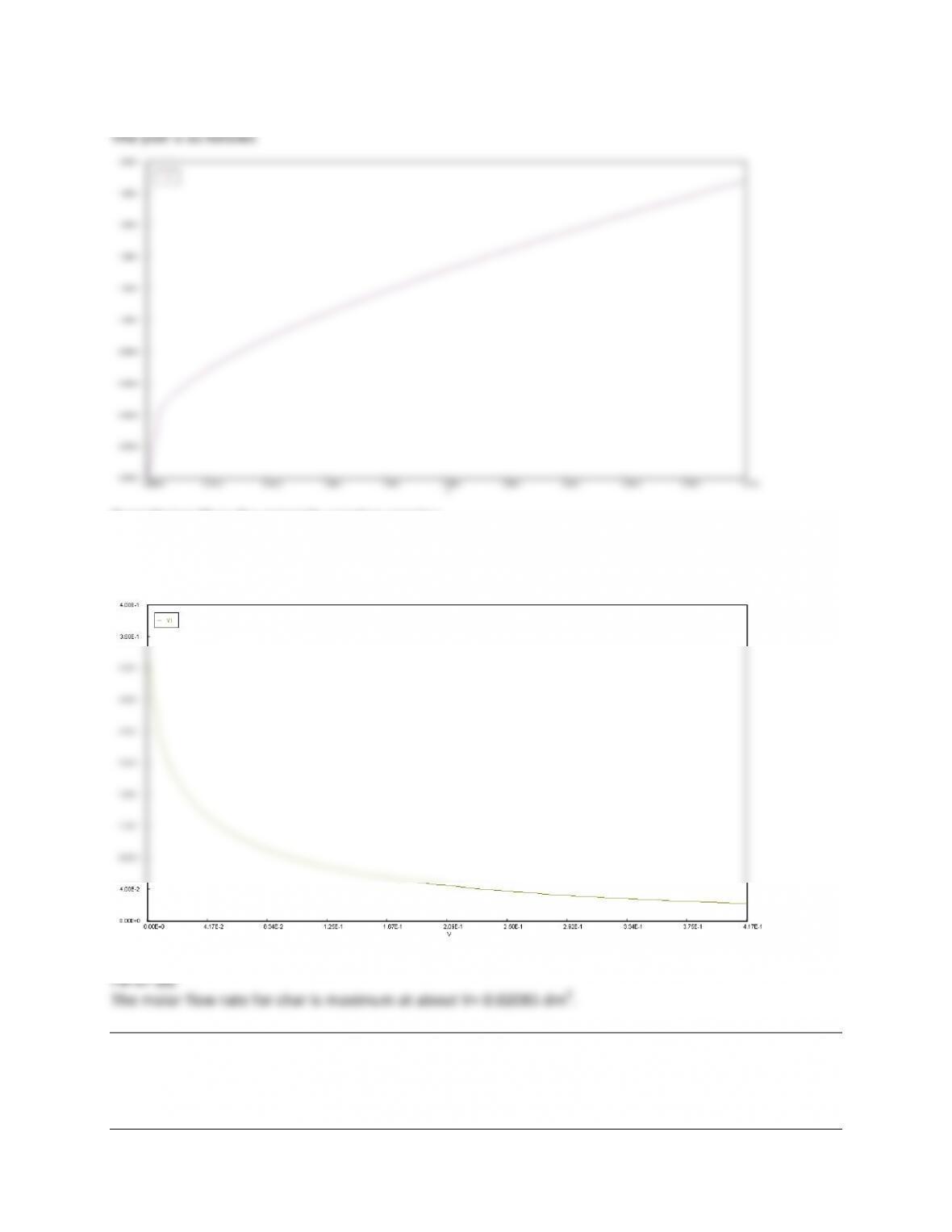
For$t$=$0$to$t$=$0.35$sec,$PSSH$is$not$valid$as$steady$state$not$reached.$