6-15$
P6-6$(a))Continued$
Rename$
Transport$out$the$sides$of$the$reactor:$
RA$=$kACA$=
$
–rA$=$rB$=1/2$rC$
Combine$and$solve$in$Polymath$code:$
See$Polymath$program$P6-6-a.pol.$
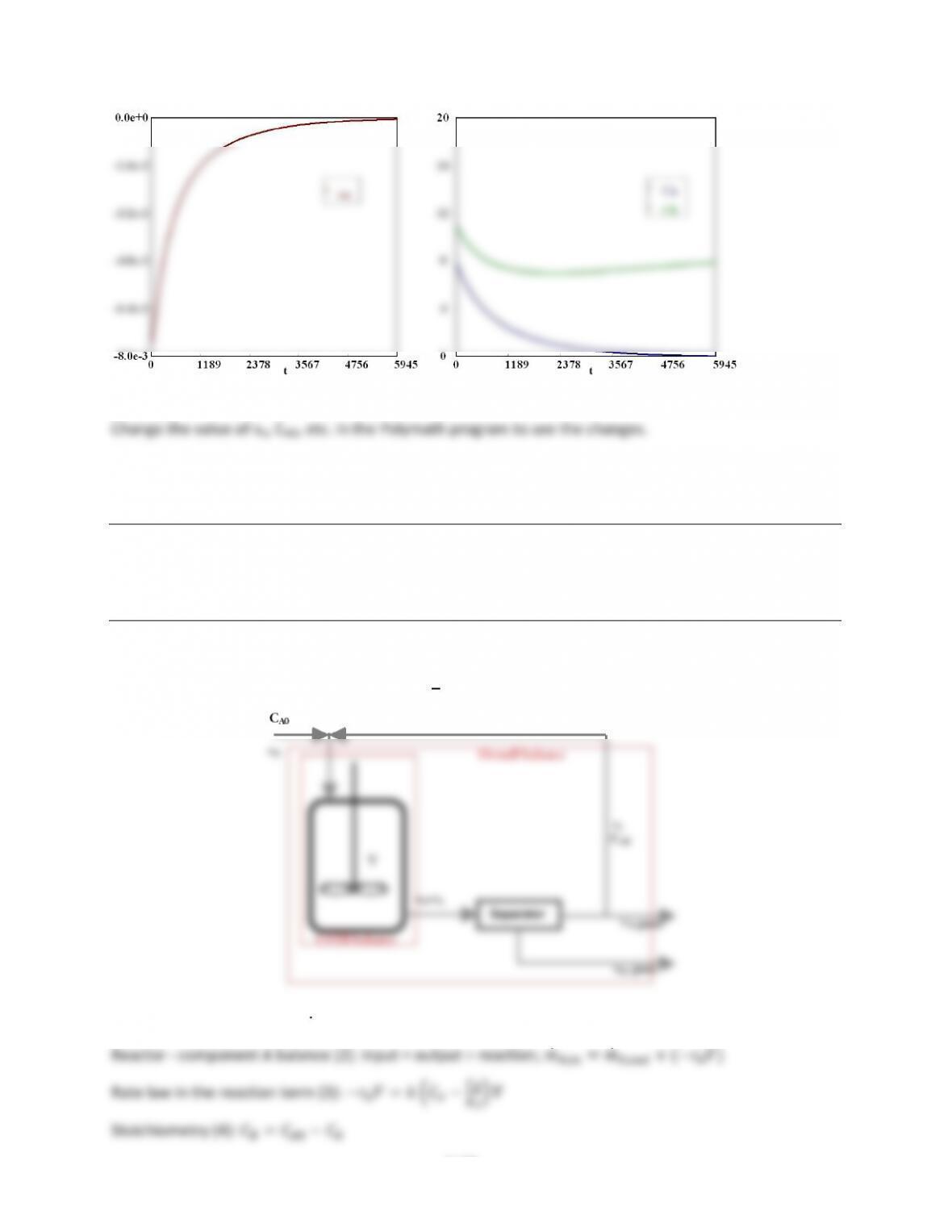
POLYMATH)Results$
Calculated)values)of)the)DEQ)variables$
Variable initial value minimal value maximal value final value$
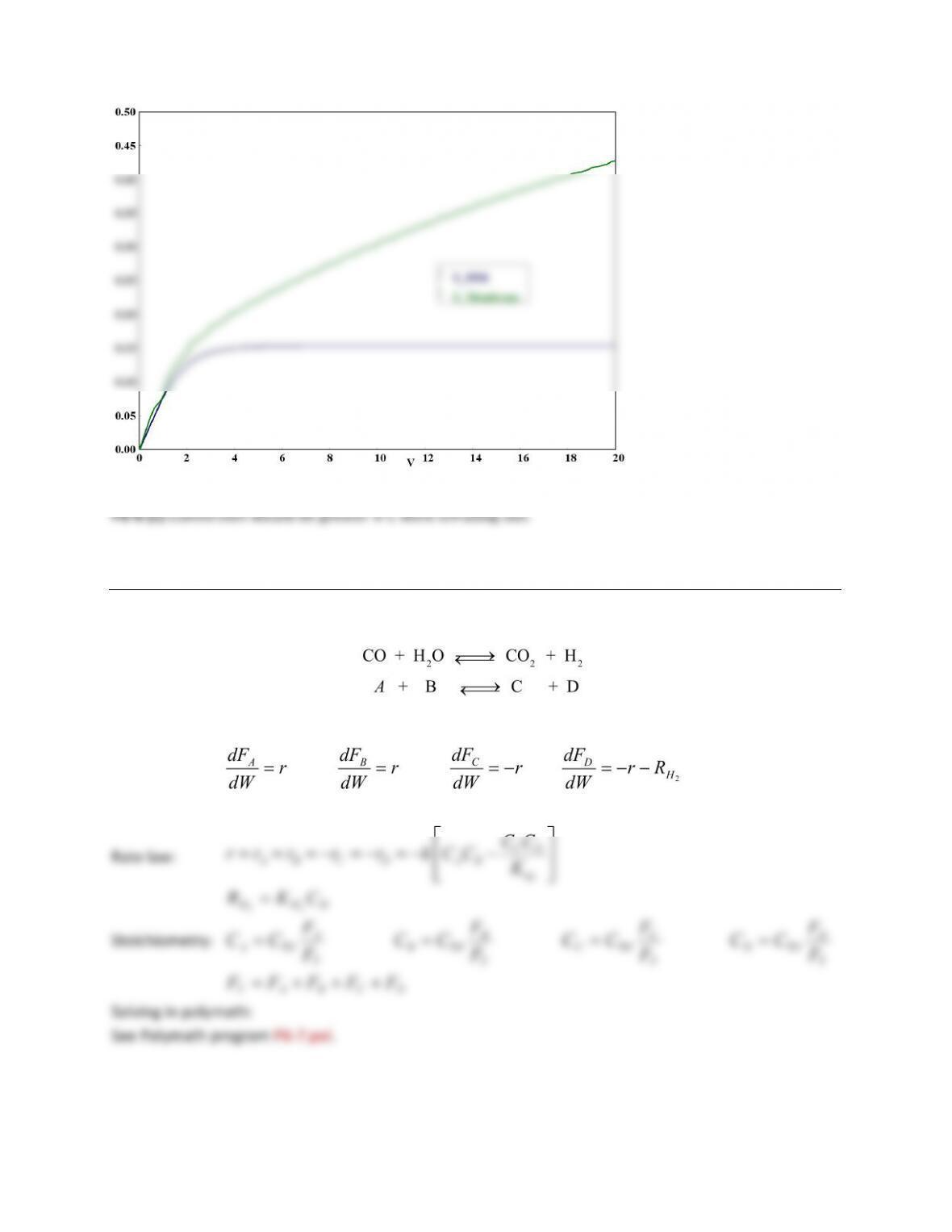
v 0 0 20 20 $
Fa 100 57.210025 100 57.210025$
Fb 0 0 9.0599877 1.935926 $
Fc 0 0 61.916043 61.916043$
Rb 0 0 2.9904791 0.6396478$
Fao 100 100 100 100 $
X 0 0 0.4278998 0.4278998$
$
ODE)Report)(RKF45)$
Differential equations as entered by the user$
[1] d(Fa)/d(v) = ra - Ra$
$
Explicit equations as entered by the user$
[1] Kc = 0.01$
[2] Ft = Fa+ Fb+ Fc$
[3] Co = 1$