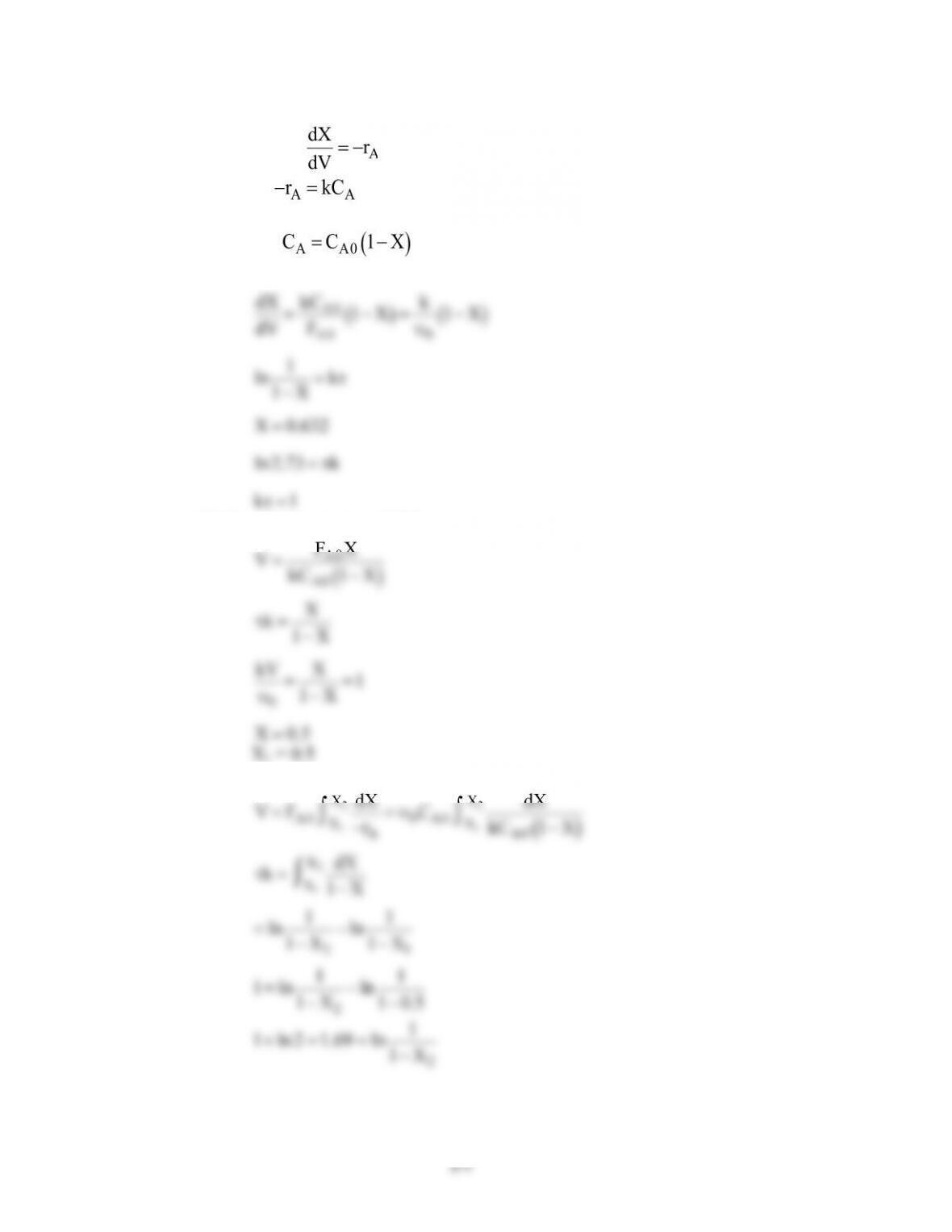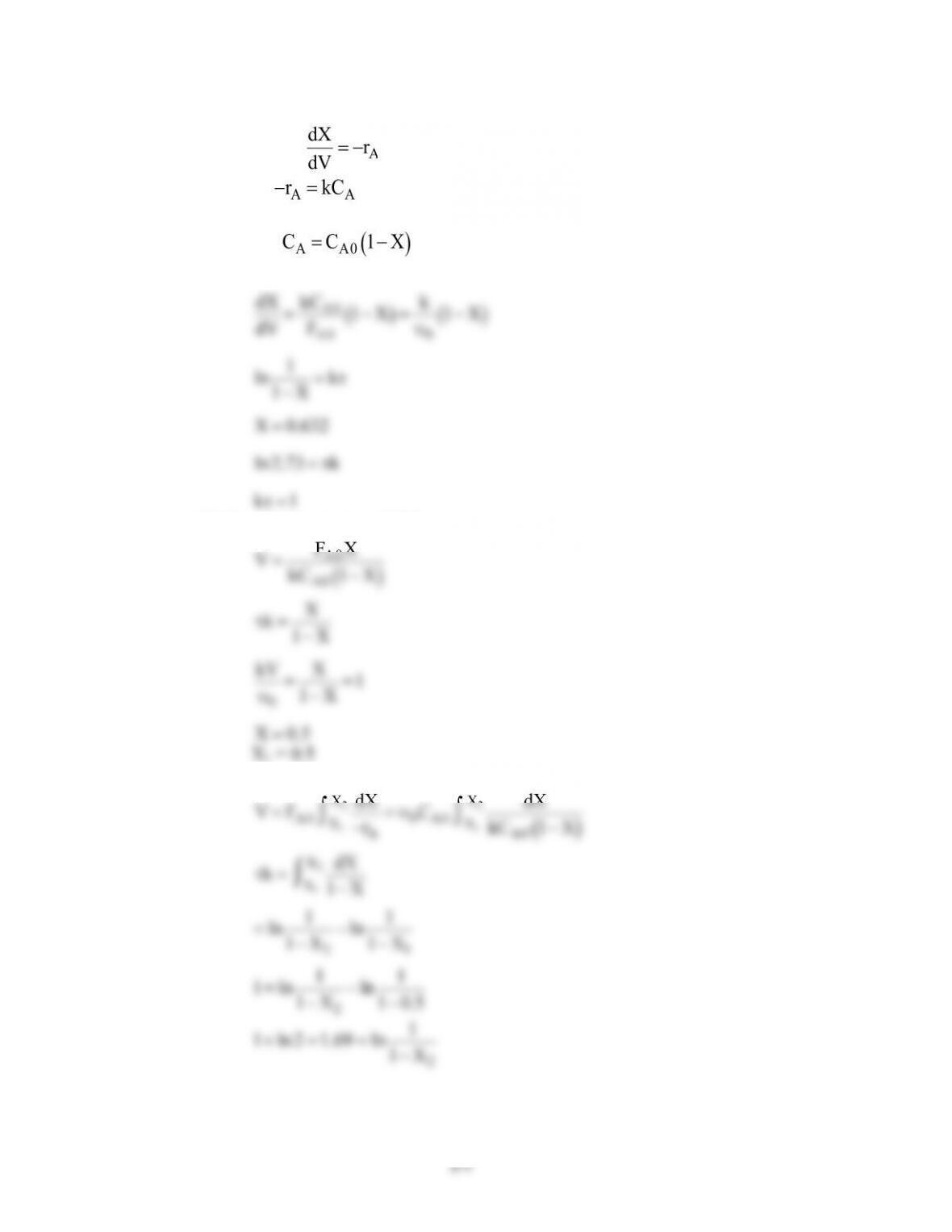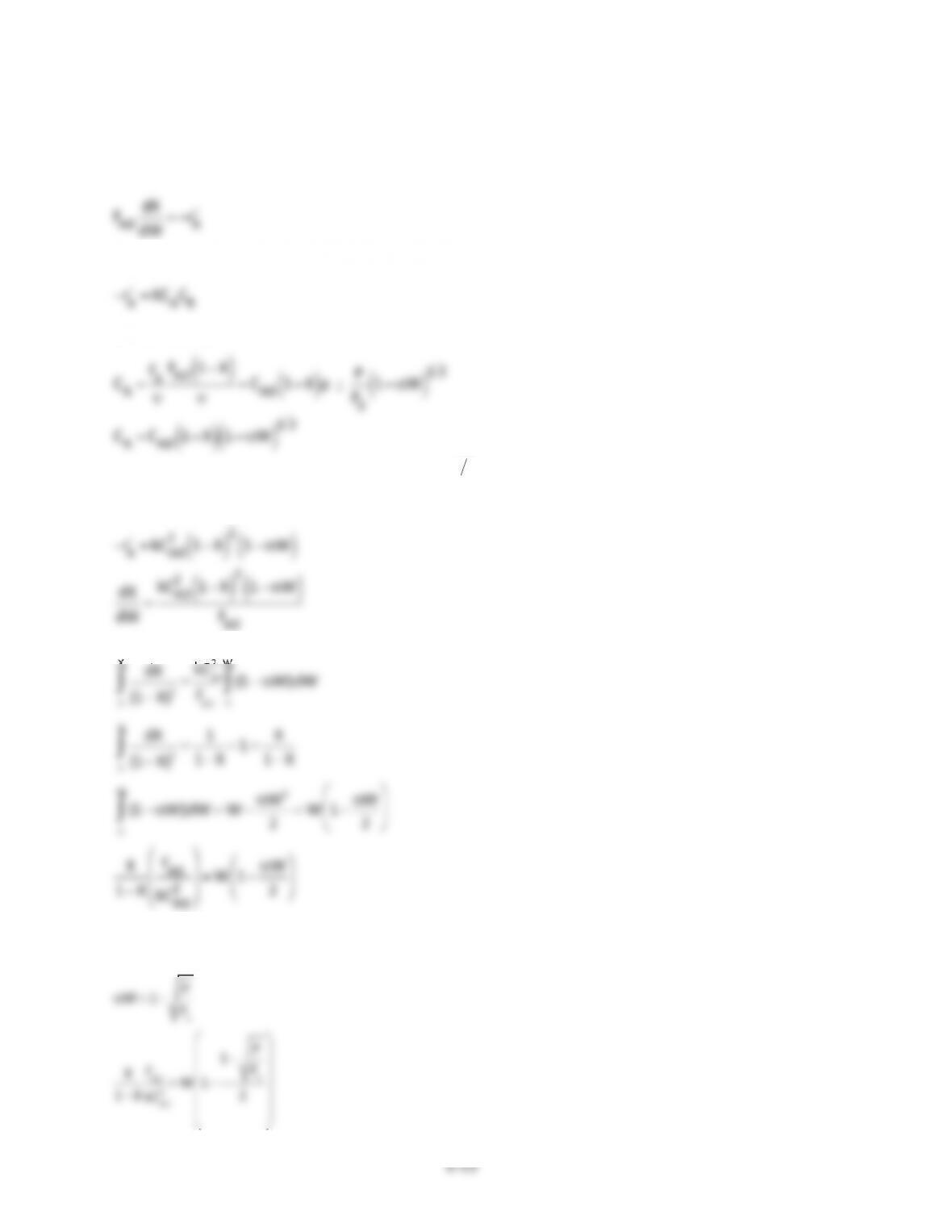5-2$
P5-1)(d))Example)5-6$Continued$
$
Therefore$there$is$no$change.$
$
P5-1)(e))Example)5-7$)
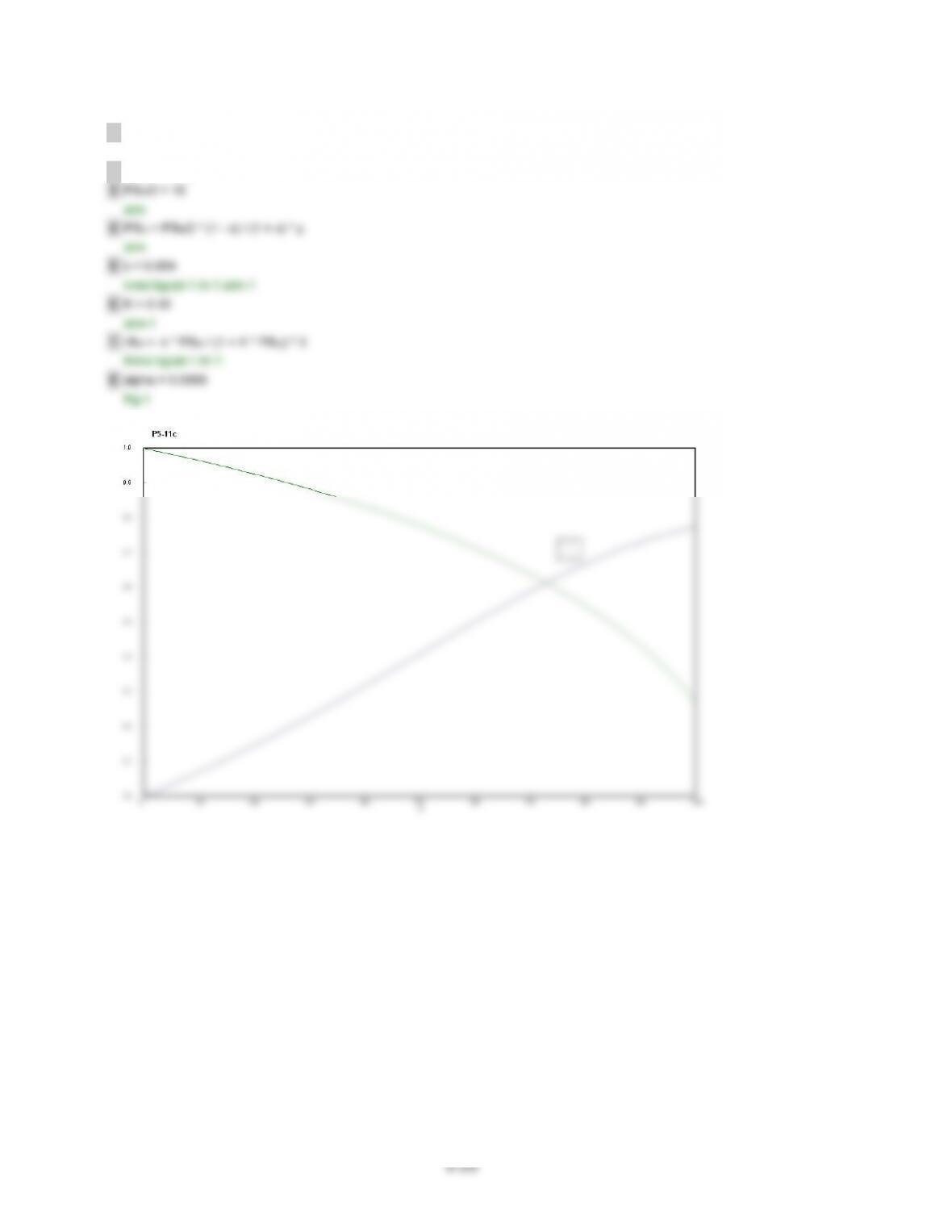
(i)$X$increases$and$f$decreases$with$an$increase$in$k’$for$the$same$W,$while$p$remains$unchanged,$and$
vice$versa.$This$is$an$expected$observation,$because$as$k’$increases,$the$rate$of$the$reaction$increases,$
$
P5-1)(f))Example)5-8$)
Individualized$solution$
$
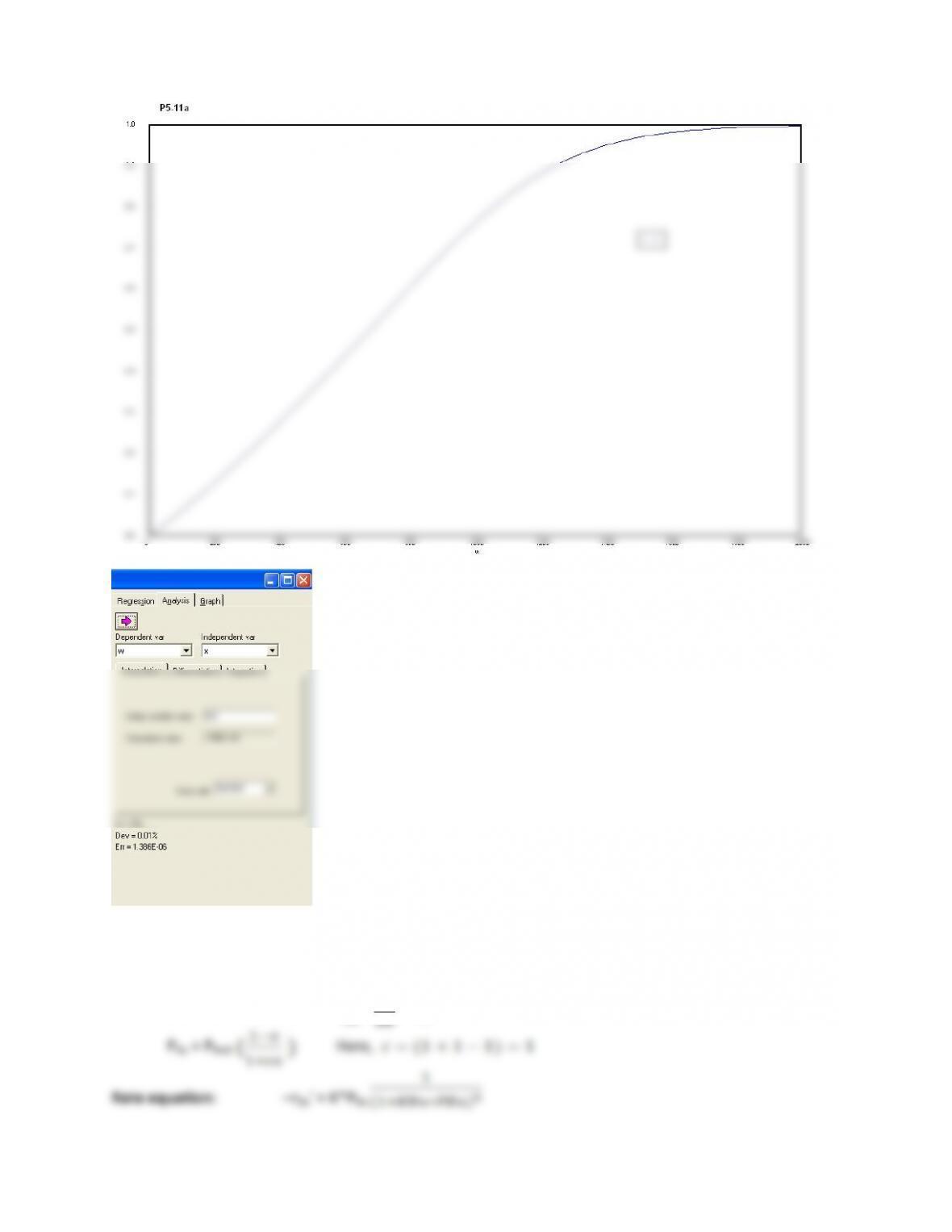
P5-1)(g))Example)5-3,)Using)ASPEN,)we)get$(Refer$to$Aspen$Program$P5-2g$from$polymath$CD)$
(1) At$1000K,$for$the$same$PFR$volume$we$get$only$6.2%$conversion.$While$at$1200K,$we$get$a$
conversion$of$nearly$100%.$This$is$because$the$value$of$reaction$constant$‘k’$varies$rapidly$
(2) Earlier$for$an$activation$energy$of$82$kcal/mol$we$got$approx.$81%$conversion.$For$activation$
energy$of$74$kcal/mol$keeping$the$PFR$volume$the$same$we$get$a$conversion$of$71.1%.$
$
P5-1)(h)$Individualized$solution.$
$
P5-1)(i)$Individualized$solution$
)
$
P5-2)The$key$for$decoding$the$algorithm$to$arrive$at$a$numerical$score$for$the$Interaction$Computer$
Games$(ICGs)$is$given$at$the$front$of$this$Solutions$Manual.$
$