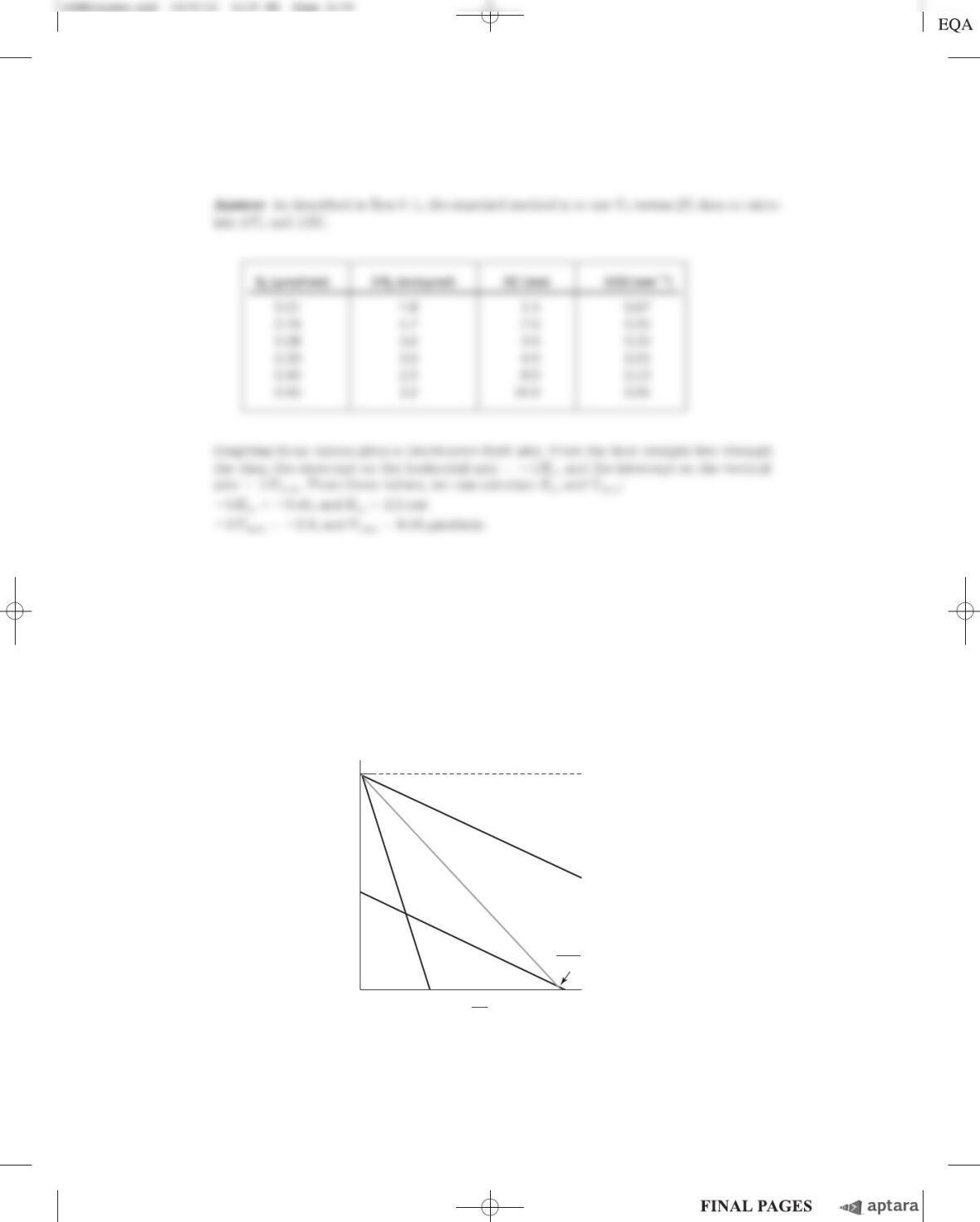
Chapter 6 Enzymes S-67
(b) In another experiment, with [E
t
] at 1 n
M
and [HAPPY] at 30
M
, the researchers find that V
0
300 n
M
s
1
. What is the measured K
m
of happyase* for its substrate HAPPY? (Include appropriate
units.)
(c) Further research shows that the purified happyase* used in the first two experiments was actually
contaminated with a reversible inhibitor called ANGER. When ANGER is carefully removed from
the happyase* preparation, and the two experiments repeated, the measured V
max
in (a) is in-
creased to 4.8
M
s
1
, and the measured K
m
in (b) is now 15
M
. For the inhibitor ANGER, calcu-
late the values of ␣and ␣.
(d) Based on the information given above, what type of inhibitor is ANGER?
10. Applying the Michaelis-Menten Equation II Another enzyme is found that catalyzes the reaction
A88z
y88 B
Researchers find that the K
m
for the substrate A is 4
M
, and the k
cat
is 20 min
1
.
(a) In an experiment, [A] 6 m
M
, and the initial velocity, V
0
was 480 n
M
min
1
. What was the [E
t
] used
in the experiment?
(b) In another experiment, [E
t
] 0.5
M
, and the measured V
0
5
M
min
1
. What was the [A] used
in the experiment?
(c) The compound Z is found to be a very strong competitive inhibitor of the enzyme, with an ␣of
10. In an experiment with the same [E
t
] as in part (a), but a different [A], an amount of Z is
added that reduces the rate V
0
to 240 n
M
min
1
. What is the [A] in this experiment?
(d) Based on the kinetic parameters given above, has this enzyme evolved to achieve catalytic
perfection? Explain your answer briefly, using the kinetic parameter(s) that define catalytic
perfection.
Answer