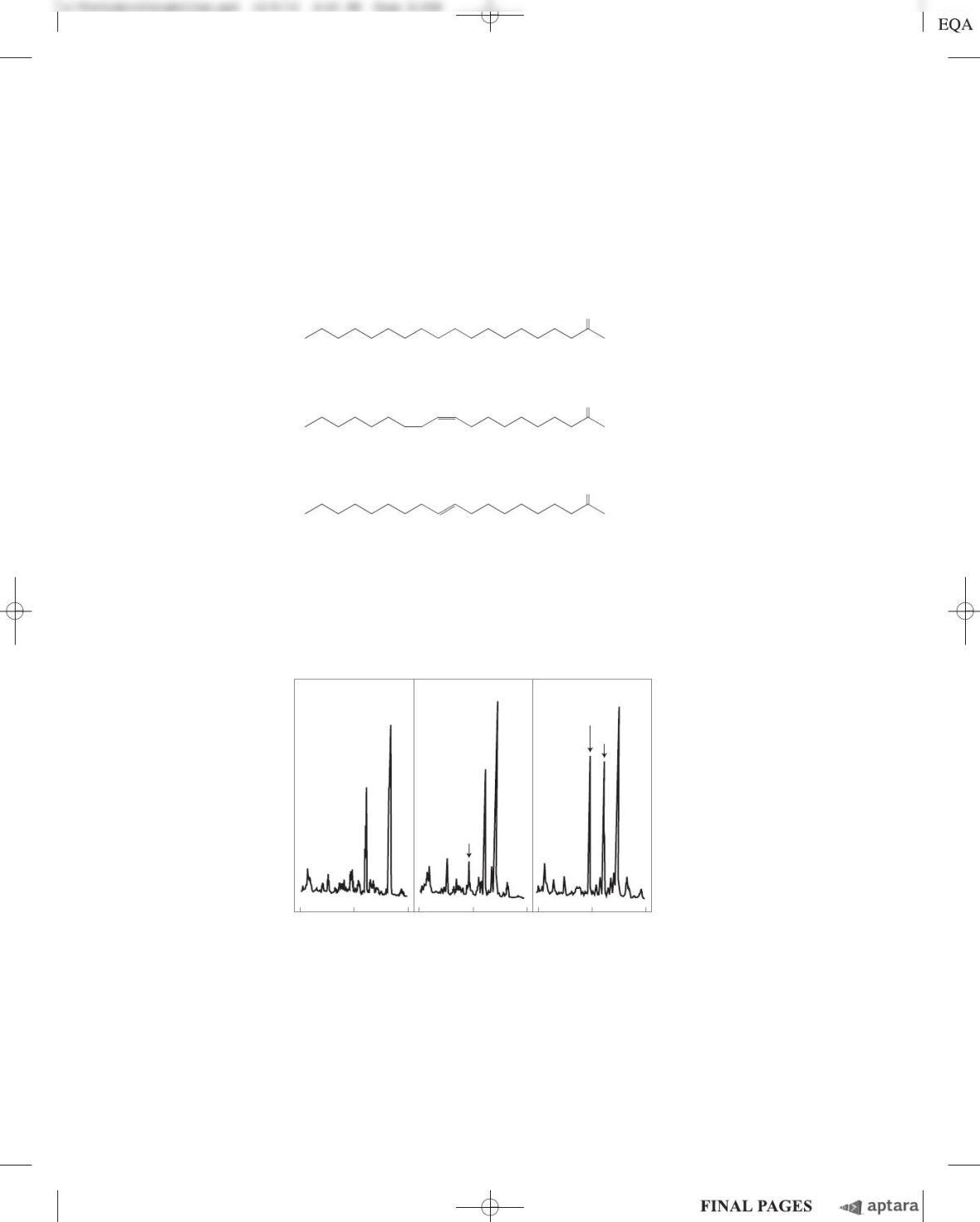
Chapter 17 Fatty Acid Catabolism S-209
(a) Why did Yu and colleagues need to use CoA derivatives rather than the free fatty acids in these
experiments?
(b) Why were no lower molecular weight CoA derivatives found in the reaction with stearoyl-CoA?
(c) How many rounds of oxidation would be required to convert the oleoyl-CoA and the elaidoyl-
CoA to cis-
5
-tetradecenoyl-CoA and trans-
5
-tetradecenoyl-CoA, respectively?
There are two forms of the enzyme acyl-CoA dehydrogenase (see Fig. 17–8a): long-chain
acyl-CoA dehydrogenase (LCAD) and very-long-chain acyl-CoA dehydrogenase (VLCAD). Yu and
coworkers measured the kinetic parameters of both enzymes. They used the CoA derivatives of
three fatty acids: tetradecanoyl-CoA (C
14
-CoA), cis-
5
-tetradecenoyl-CoA (c
5
C
14
-CoA), and
trans-
5
-tetradecenoyl-CoA (t
5
C
14
-CoA). The results are shown below. (See Chapter 6 for
definitions of the kinetic parameters.)
(d) For LCAD, the K
m
differs dramatically for the cis and trans substrates. Provide a plausible expla-
nation for this observation in terms of the structures of the substrate molecules. (Hint: You may
want to refer to Fig. 10–2.)
(e) The kinetic parameters of the two enzymes are relevant to the differential processing of these
fatty acids only if the LCAD or VLCAD reaction (or both) is the rate-limiting step in the path-
way. What evidence is there to support this assumption?
(f) How do these different kinetic parameters explain the different levels of the CoA derivatives
found after incubation of rat liver mitochondria with stearoyl-CoA, oleoyl-CoA, and elaidoyl-CoA
(shown in the three-panel figure)?
Yu and coworkers measured the substrate specificity of rat liver mitochondrial thioesterase, which
hydrolyzes acyl-CoA to CoA and free fatty acid (see Chapter 21). This enzyme was approximately
twice as active with C
14
-CoA thioesters as with C
18
-CoA thioesters.
(g) Other research has suggested that free fatty acids can pass through membranes. In their experi-
ments, Yu and colleagues found trans-
5
-tetradecenoic acid outside mitochondria (i.e., in the
medium) that had been incubated with elaidoyl-CoA. Describe the pathway that led to this ex-
tramitochondrial trans-
5
-tetradecenoic acid. Be sure to indicate where in the cell the various
transformations take place, as well as the enzymes that catalyze the transformations.