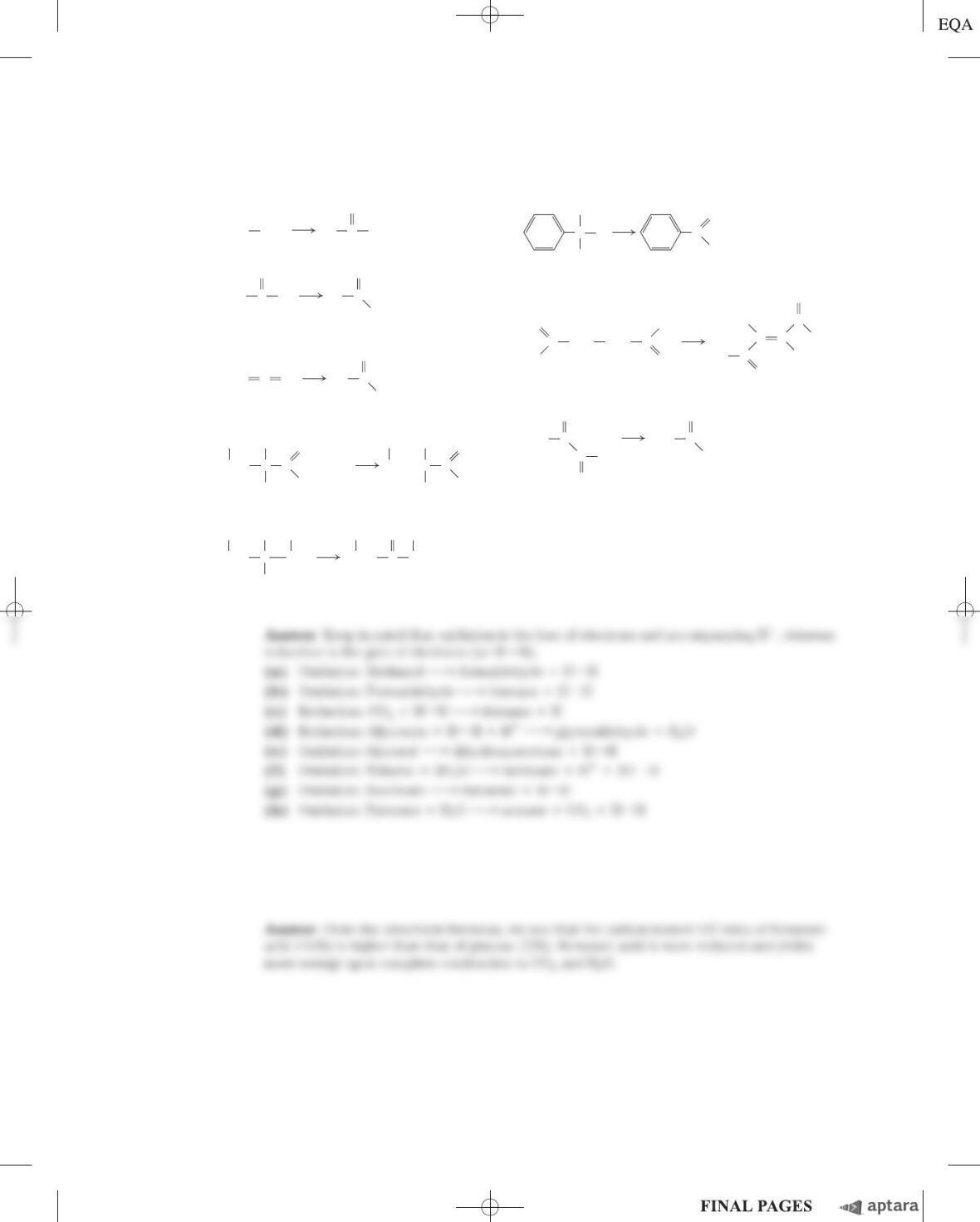
S-196 Chapter 16 The Citric Acid Cycle
35. Partitioning between the Citric Acid and Glyoxylate Cycles In an organism (such as E. coli)
that has both the citric acid cycle and the glyoxylate cycle, what determines which of these pathways
isocitrate will enter?
Data Analysis Problem
36. How the Citric Acid Cycle Was Determined The detailed biochemistry of the citric acid cycle was
determined by several researchers over a period of decades. In a 1937 article, Krebs and Johnson sum-
marized their work and the work of others in the first published description of this pathway.
The methods used by these researchers were very different from those of modern biochemistry.
Radioactive tracers were not commonly available until the 1940s, so Krebs and other researchers had
to use nontracer techniques to work out the pathway. Using freshly prepared samples of pigeon breast
muscle, they determined oxygen consumption by suspending minced muscle in buffer in a sealed flask
and measuring the volume (in L) of oxygen consumed under different conditions. They measured
levels of substrates (intermediates) by treating samples with acid to remove contaminating proteins,
then assaying the quantities of various small organic molecules. The two key observations that led
Krebs and colleagues to propose a citric acid cycle as opposed to a linear pathway (like that of gly-
colysis) were made in the following experiments.
Experiment I. They incubated 460 mg of minced muscle in 3 mL of buffer at 40 C for 150 min-
utes. Addition of citrate increased O
2
consumption by 893 L compared with samples without added
citrate. They calculated, based on the O
2
consumed during respiration of other carbon-containing
compounds, that the expected O
2
consumption for complete respiration of this quantity of citrate was
only 302 L.
Experiment II. They measured O
2
consumption by 460 mg of minced muscle in 3 mL of buffer
when incubated with citrate and/or with 1-phosphoglycerol (glycerol 1-phosphate; this was known to
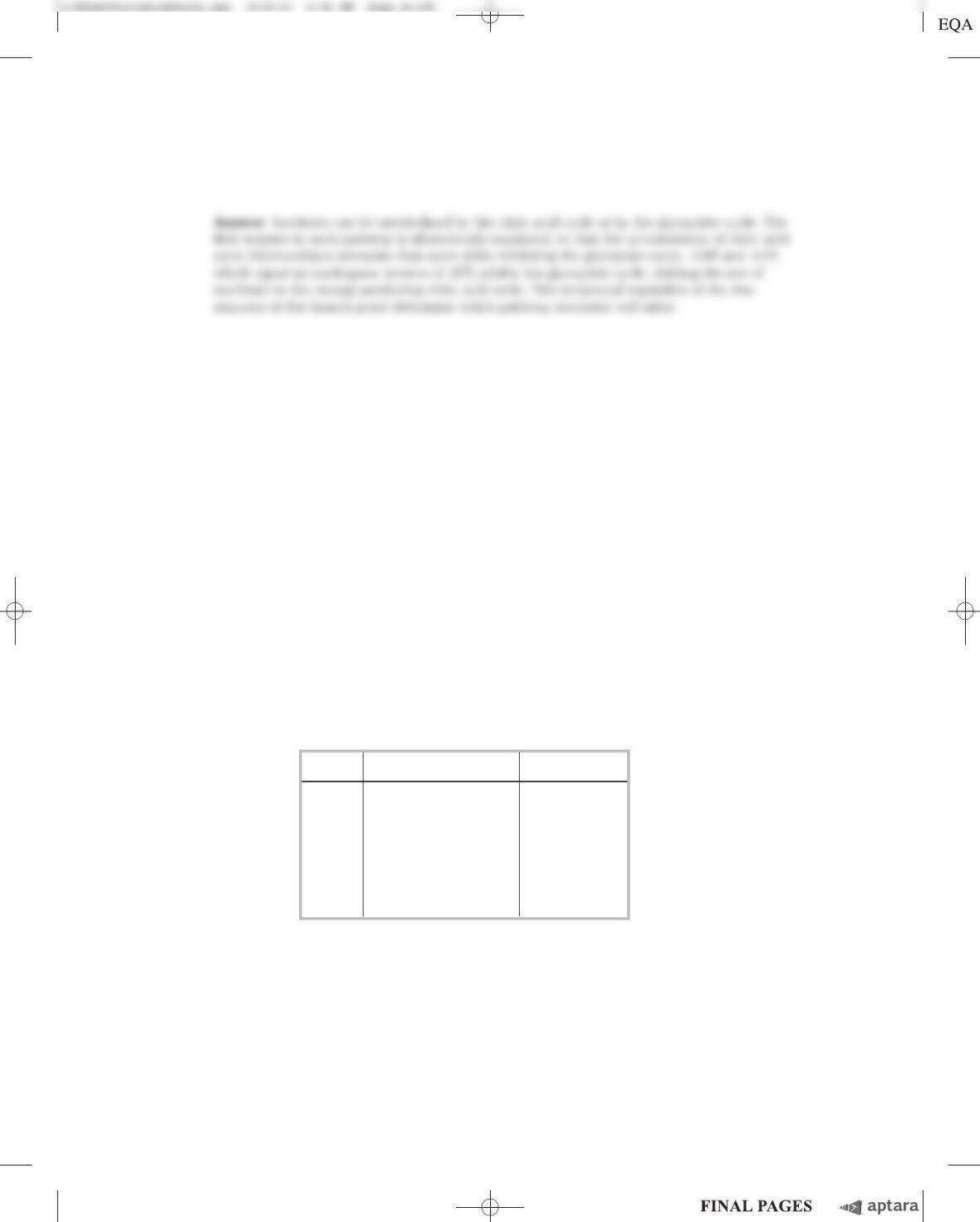
be readily oxidized by cellular respiration) at 40 C for 140 minutes. The results are shown in the
table.
Sample Substrate(s) added L O
2
absorbed
1 No extra 342
2 0.3 mL 0.2
M
1-phosphoglycerol 757
3 0.15 mL 0.02
M
citrate 431
4 0.3 mL 0.2
M
1-phosphoglycerol and
0.15 mL 0.02
M
citrate 1,385
(a) Why is O
2
consumption a good measure of cellular respiration?
(b) Why does sample 1 (unsupplemented muscle tissue) consume some oxygen?
(c) Based on the results for samples 2 and 3, can you conclude that 1-phosphoglycerol and citrate
serve as substrates for cellular respiration in this system? Explain your reasoning.
(d) Krebs and colleagues used the results from these experiments to argue that citrate was
“catalytic”—that it helped the muscle tissue samples metabolize 1-phosphoglycerol more
completely. How would you use their data to make this argument?