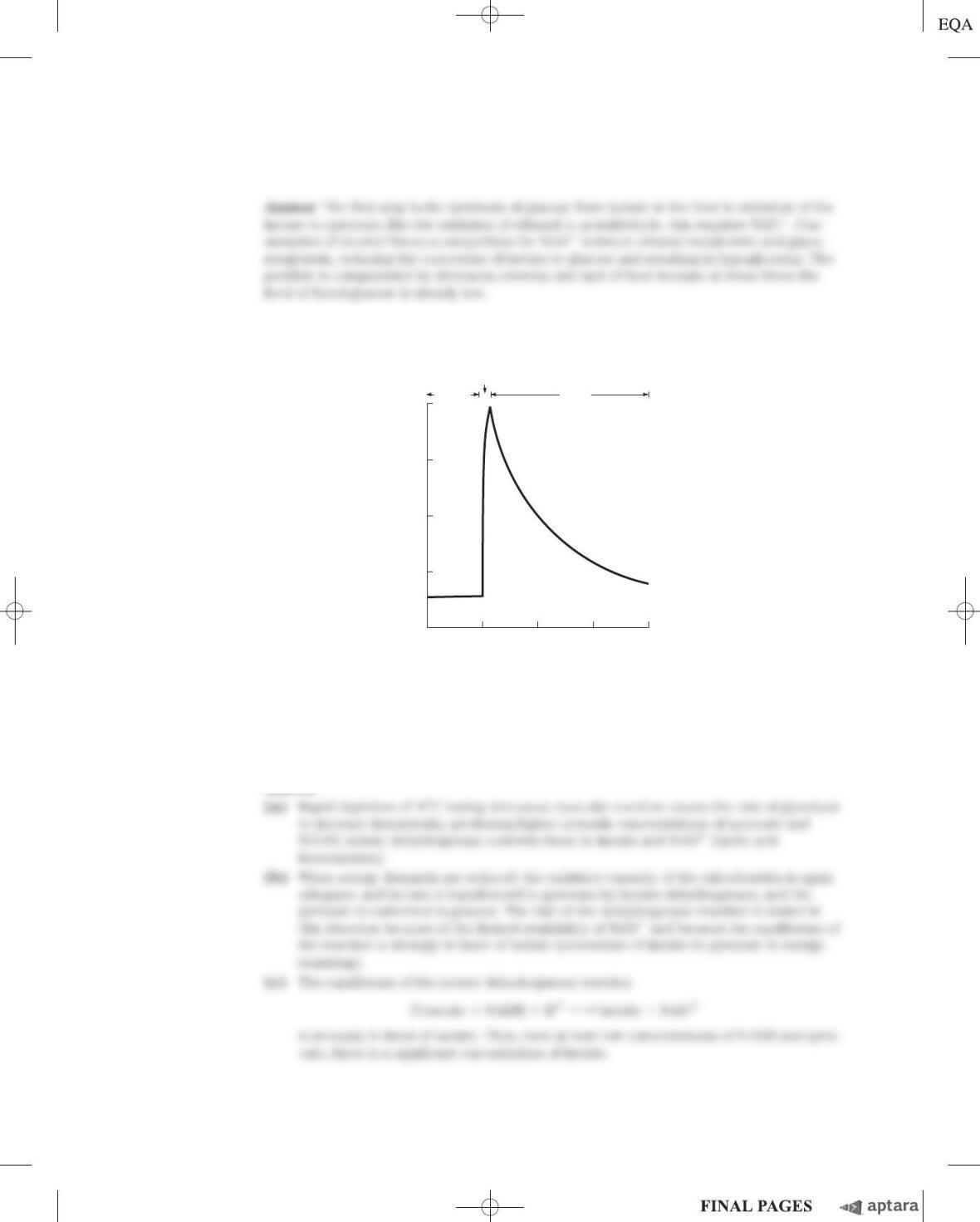
30. Role of the Pentose Phosphate Pathway If the oxidation of glucose 6-phosphate via the pentose
phosphate pathway were being used primarily to generate NADPH for biosynthesis, the other product,
ribose 5-phosphate, would accumulate. What problems might this cause?
Data Analysis Problem
31. Engineering a Fermentation System Fermentation of plant matter to produce ethanol for fuel is
one potential method for reducing the use of fossil fuels and thus the CO
2
emissions that lead to global
warming. Many microorganisms can break down cellulose then ferment the glucose to ethanol. How-
ever, many potential cellulose sources, including agricultural residues and switchgrass, also contain
substantial amounts of arabinose, which is not as easily fermented.
Escherichia coli is capable of fermenting arabinose to ethanol, but it is not naturally tolerant of
high ethanol levels, thus limiting its utility for commercial ethanol production. Another bacterium,
Zymomonas mobilis, is naturally tolerant of high levels of ethanol but cannot ferment arabinose.
Deanda, Zhang, Eddy, and Picataggio (1996) described their efforts to combine the most useful features
of these two organisms by introducing the E. coli genes for the arabinose-metabolizing enzymes into
Z. mobilis.
(a) Why is this a simpler strategy than the reverse: engineering E. coli to be more ethanol-tolerant?
Deanda and colleagues inserted five E. coli genes into the Z. mobilis genome: araA, coding for
L
-arabinose isomerase, which interconverts
L
-arabinose and
L
-ribulose; araB,
L
-ribulokinase, which uses
ATP to phosphorylate
L
-ribulose at C-5; araD,
L
-ribulose 5-phosphate epimerase, which interconverts
L
-ribulose 5-phosphate and
L
-xylulose 5-phosphate; talB, transaldolase; and tktA, transketolase.
(b) For each of the three ara enzymes, briefly describe the chemical transformation it catalyzes and,
where possible, name an enzyme discussed in this chapter that carries out an analogous reaction.
The five E. coli genes inserted in Z. mobilis allowed the entry of arabinose into the nonoxidative
phase of the pentose phosphate pathway (Fig. 14–23), where it was converted to glucose 6-phosphate
and fermented to ethanol.
(c) The three ara enzymes eventually converted arabinose into which sugar?
(d) The product from part (c) feeds into the pathway shown in Figure 14–23. Combining the five
E. coli enzymes listed above with the enzymes of this pathway, describe the overall pathway for
the fermentation of 6 molecules of arabinose to ethanol.
(e) What is the stoichiometry of the fermentation of 6 molecules of arabinose to ethanol and CO
2
?
How many ATP molecules would you expect this reaction to generate?
(f) Z. mobilis uses a slightly different pathway for ethanol fermentation from the one described in
this chapter. As a result, the expected ATP yield is only 1 ATP per molecule of arabinose.
Although this is less beneficial for the bacterium, it is better for ethanol production. Why?