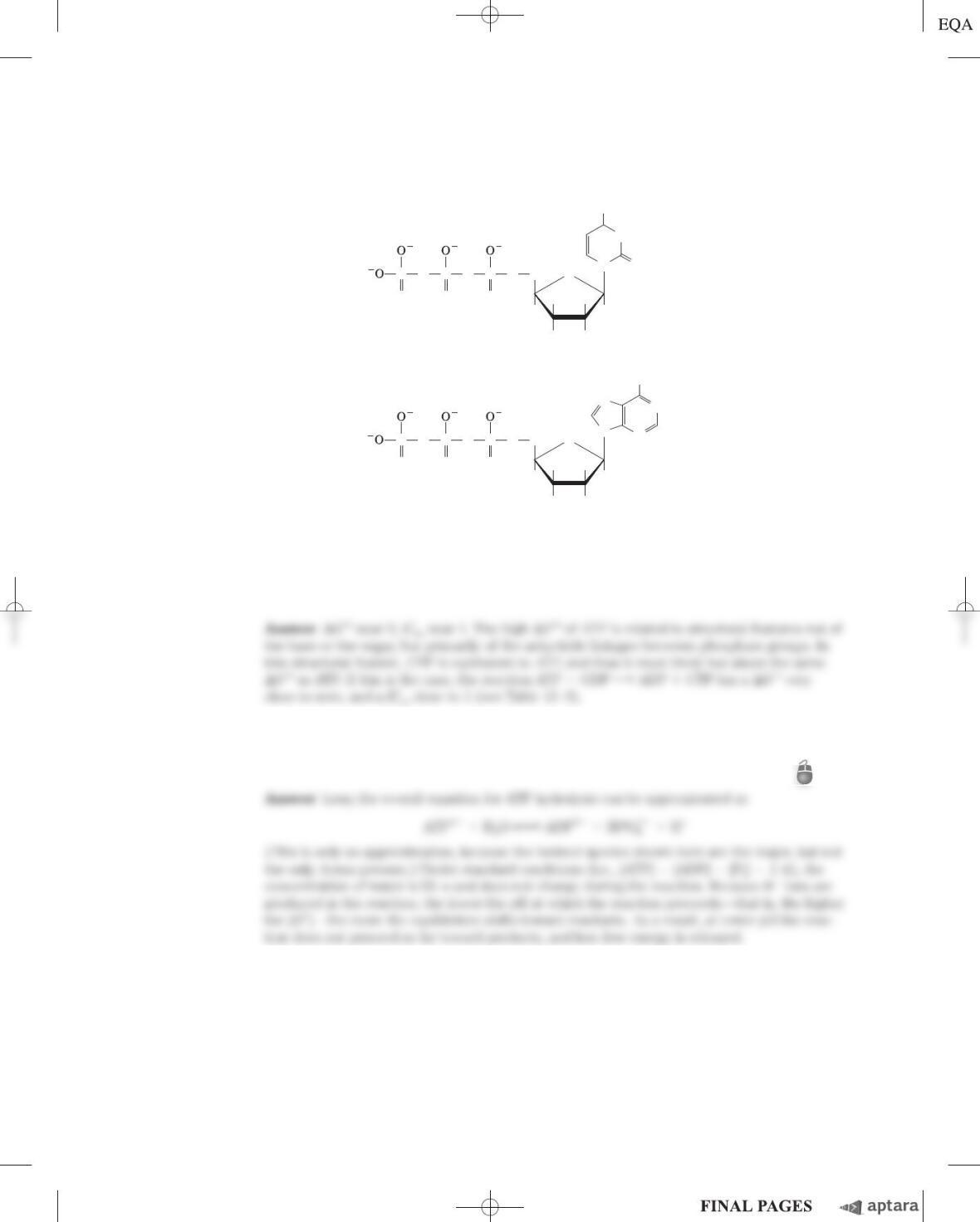
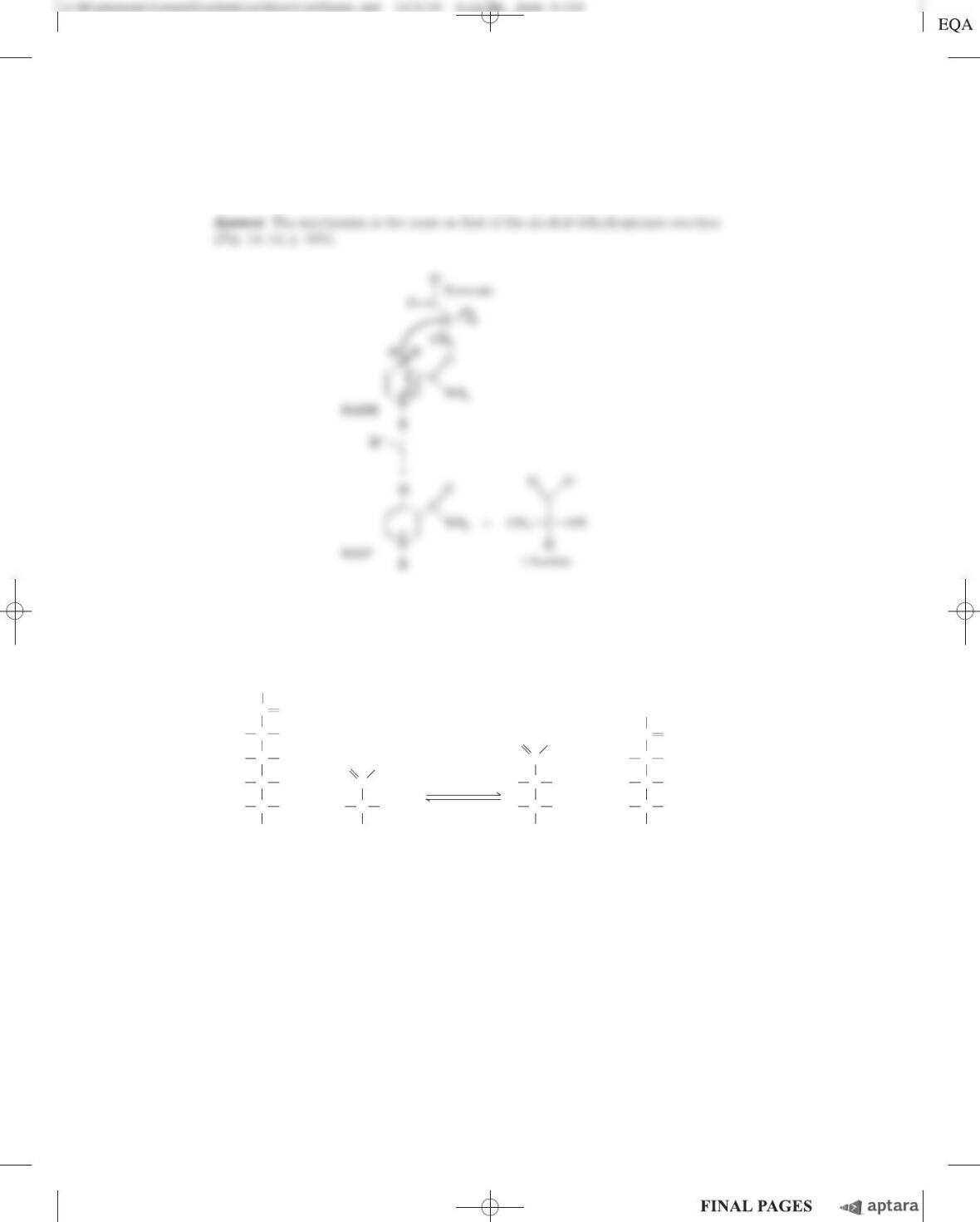
Chapter 13 Bioenergetics and Biochemical Reaction Types S-147
11. Strategy for Overcoming an Unfavorable Reaction: ATP-Dependent Chemical Coupling The
phosphorylation of glucose to glucose 6-phosphate is the initial step in the catabolism of glucose. The direct
phosphorylation of glucose by P
i
is described by the equation
Glucose P
i
88n glucose 6-phosphate H
2
OG 13.8 kJ/mol
(a) Calculate the equilibrium constant for the above reaction at 37 C. In the rat hepatocyte the
physiological concentrations of glucose and P
i
are maintained at approximately 4.8 m
M
. What is
the equilibrium concentration of glucose 6-phosphate obtained by the direct phosphorylation of
glucose by P
i
? Does this reaction represent a reasonable metabolic step for the catabolism of
glucose? Explain.
(b) In principle, at least, one way to increase the concentration of glucose 6-phosphate is to drive
the equilibrium reaction to the right by increasing the intracellular concentrations of glucose and
P
i
. Assuming a fixed concentration of P
i
at 4.8 m
M
, how high would the intracellular concentra-
tion of glucose have to be to give an equilibrium concentration of glucose 6-phosphate of 250 m
M
(the normal physiological concentration)? Would this route be physiologically reasonable, given
that the maximum solubility of glucose is less than 1
M
?
(c) The phosphorylation of glucose in the cell is coupled to the hydrolysis of ATP; that is, part of the
free energy of ATP hydrolysis is used to phosphorylate glucose:
(1) Glucose P
i
88n glucose 6-phosphate H
2
OG 13.8 kJ/mol
(2) ATP H
2
O 88n ADP P
i
G 30.5 kJ/mol
Sum: Glucose ATP 88n glucose 6-phosphate ADP
Calculate K
eq
at 37 C for the overall reaction. For the ATP-dependent phosphorylation of glu-
cose, what concentration of glucose is needed to achieve a 250 m
M
intracellular concentration of
glucose 6-phosphate when the concentrations of ATP and ADP are 3.38 m
M
and 1.32 m
M
, respec-
tively? Does this coupling process provide a feasible route, at least in principle, for the phospho-
rylation of glucose in the cell? Explain.
(d) Although coupling ATP hydrolysis to glucose phosphorylation makes thermodynamic sense, we
have not yet specified how this coupling is to take place. Given that coupling requires a common
intermediate, one conceivable route is to use ATP hydrolysis to raise the intracellular concentra-
tion of P
i
and thus drive the unfavorable phosphorylation of glucose by P
i
. Is this a reasonable
route? (Think about the solubility products of metabolic intermediates.)
(e) The ATP-coupled phosphorylation of glucose is catalyzed in hepatocytes by the enzyme glucoki-
nase. This enzyme binds ATP and glucose to form a glucose-ATP-enzyme complex, and the phos-
phoryl group is transferred directly from ATP to glucose. Explain the advantages of this route.