Morini, Bassoli, and Temussi (2005) used computer-based methods (often referred to as “in
silico” methods) to model the binding of sweet molecules to the sweet receptor.
(b) Why is it useful to have a computer model to predict the sweetness of molecules, instead of a
human- or animal-based taste assay?
In earlier work, Schallenberger and Acree (1967) had suggested that all sweet molecules include
an “AH-B” structural group, in which “A and B are electronegative atoms separated by a distance of
greater than 2.5 Å [0.25 nm] but less than 4 Å [0.4 nm]. H is a hydrogen atom attached to one of the
electronegative atoms by a covalent bond” (p. 481).
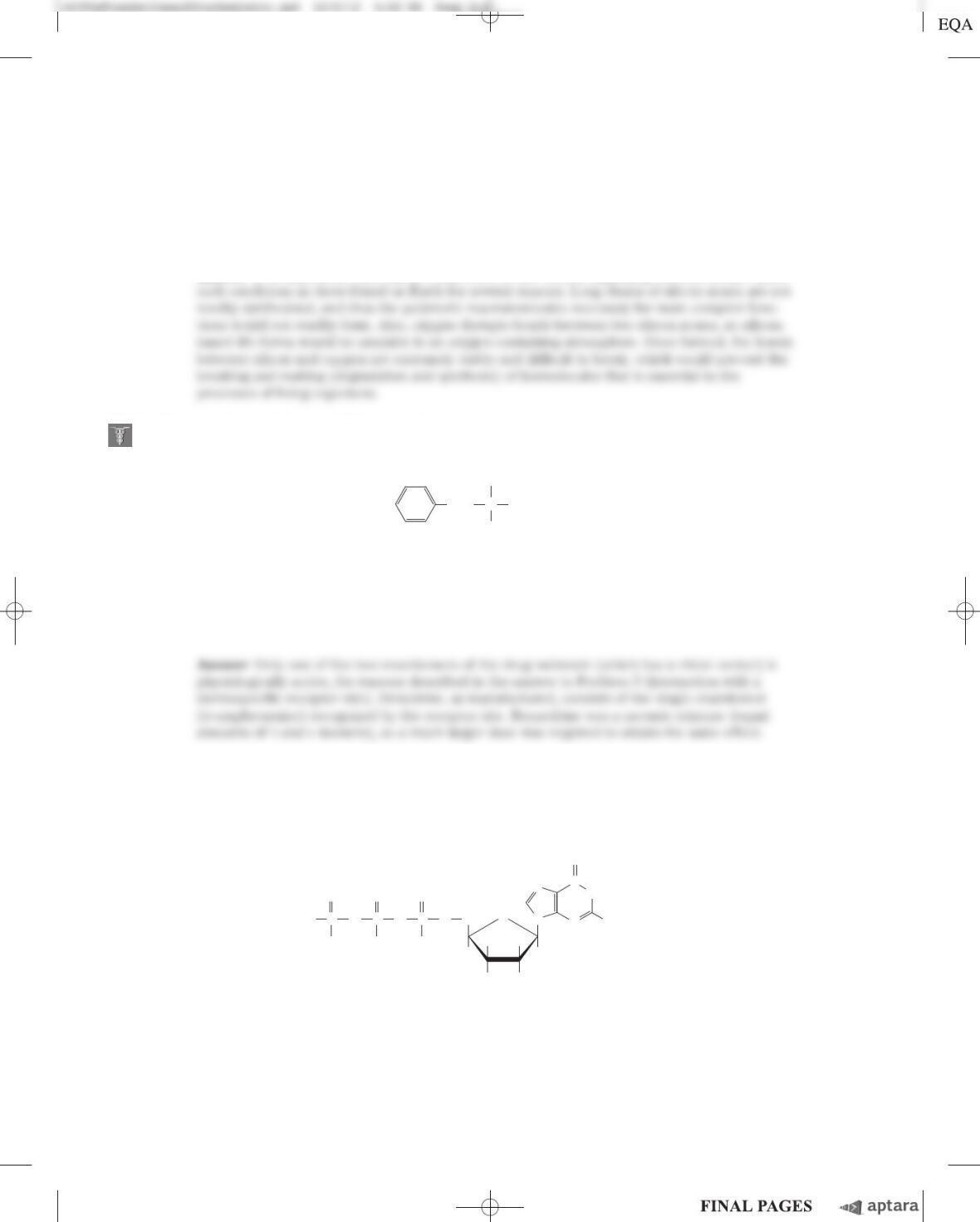
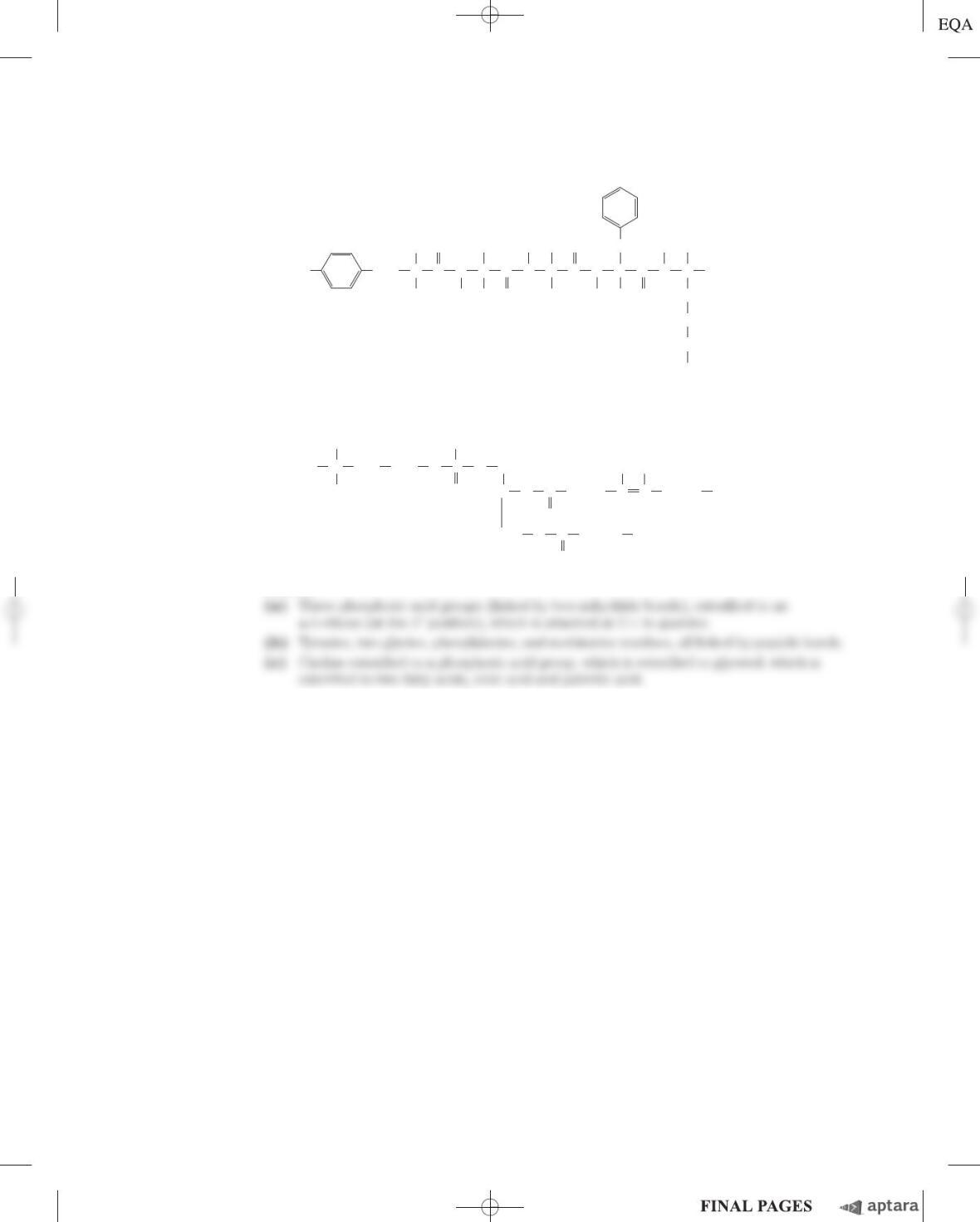
(c) Given that the length of a “typical” single bond is about 0.15 nm, identify the AH-B group(s) in
each of the molecules shown above.
(d) Based on your findings from (c), give two objections to the statement that “molecules containing
an AH-B structure will taste sweet.”
(e) For two of the molecules shown above, the AH-B model can be used to explain the difference in
MRS and G. Which two molecules are these, and how would you use them to support the AH-B
model?
(f) Several of the molecules have closely related structures but very different MRS and Gvalues.
Give two such examples, and use these to argue that the AH-B model is unable to explain the
observed differences in sweetness.
In their computer-modeling study, Morini and coauthors used the three-dimensional structure of
the sweet receptor and a molecular dynamics modeling program called GRAMM to predict the Gof
binding of sweet molecules to the sweet receptor. First, they “trained” their model—that is, they re-
fined the parameters so that the Gvalues predicted by the model matched the known Gvalues for
one set of sweet molecules (the “training set”). They then “tested” the model by asking it to predict
the Gvalues for a new set of molecules (the “test set”).
(g) Why did Morini and colleagues need to test their model against a different set of molecules from
the set it was trained on?
(h) The researchers found that the predicted Gvalues for the test set differed from the actual val-
ues by, on average, 1.3 kcal/mol. Using the values given with the structures above, estimate the
resulting error in MRS values.