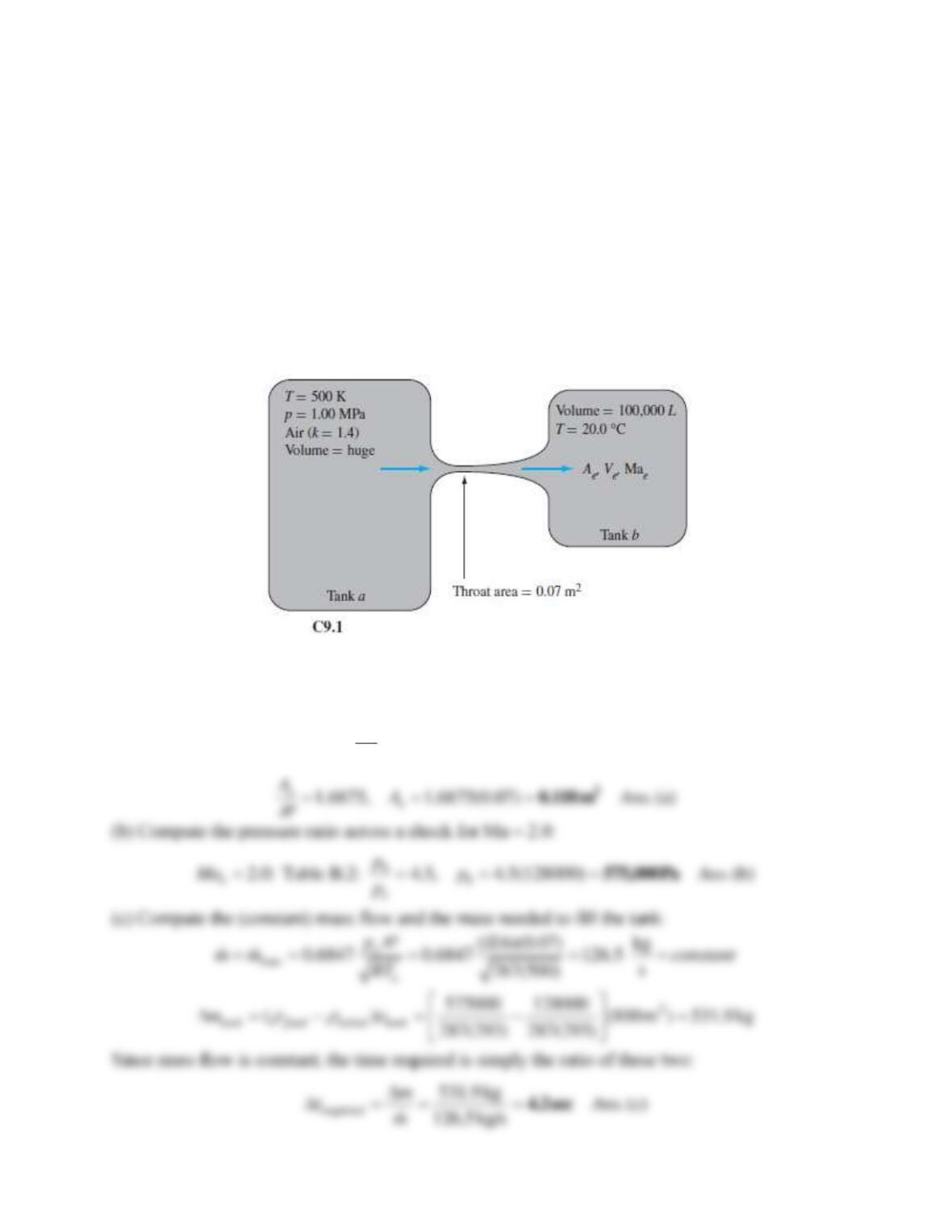
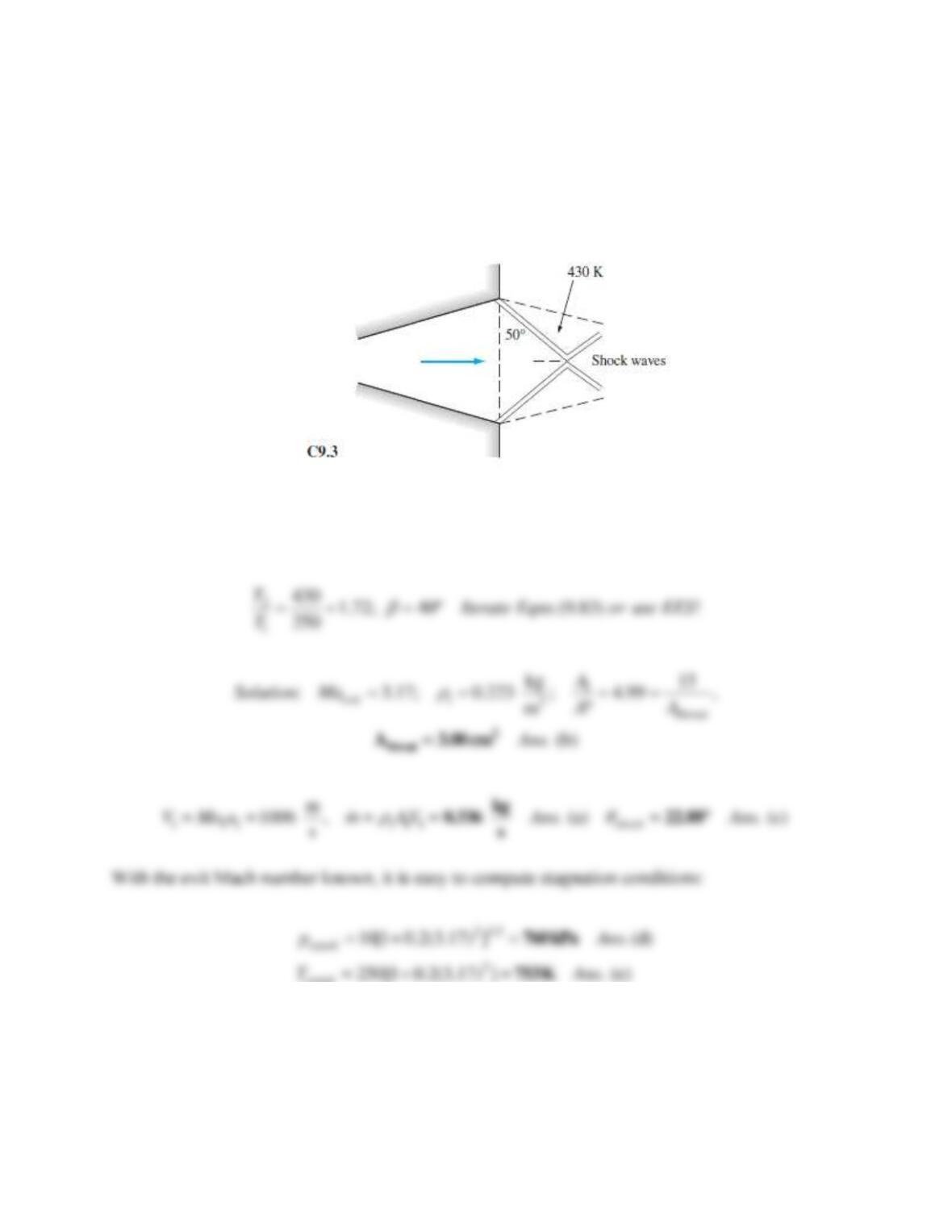
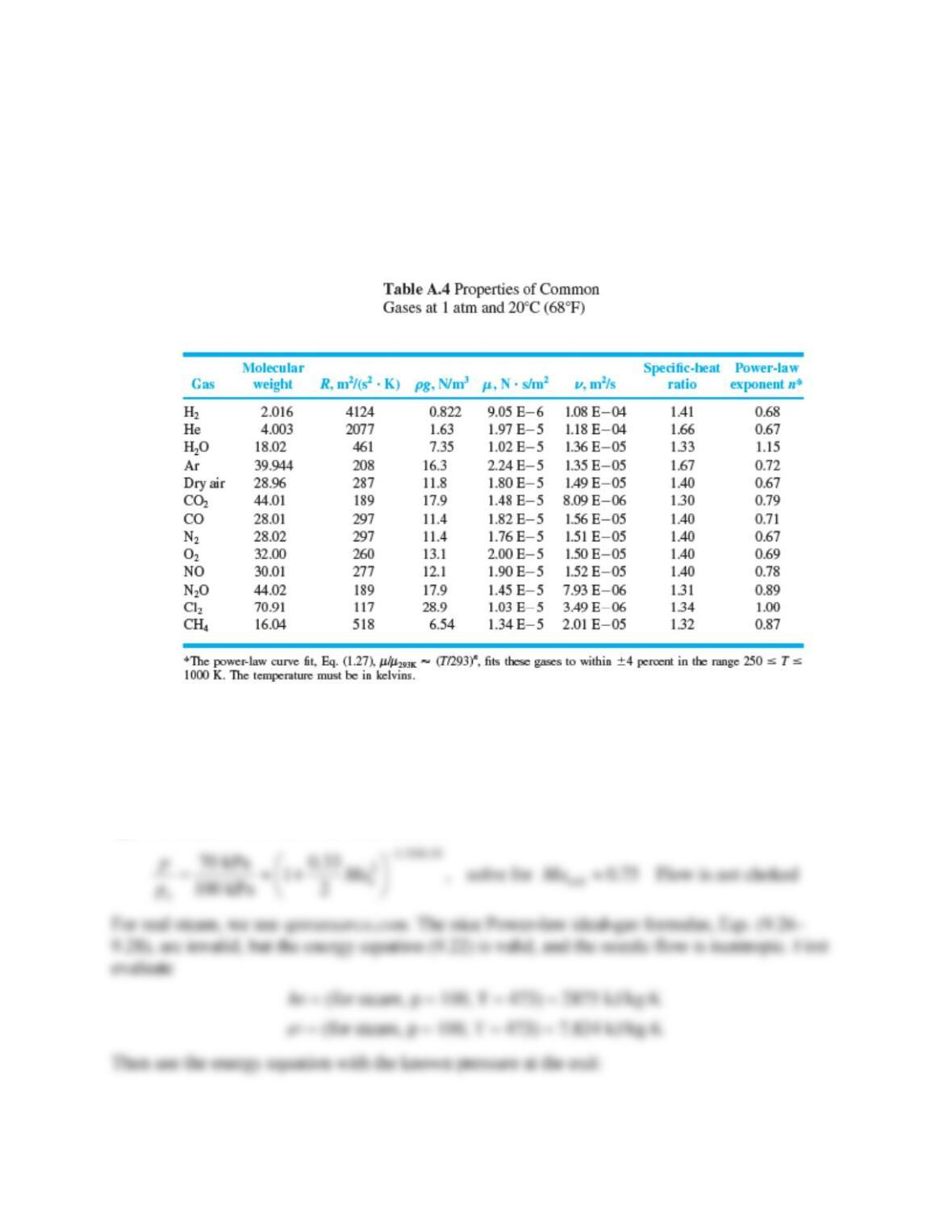
Problem 9.W3
The subject of this chapter is commonly called gas dynamics. But can liquids not perform in this
manner? Using water as an example, make a rule-of-thumb estimate of the pressure level needed
to drive a water flow at velocities comparable to the sound speed.
Solution 9.W3
Problem 9.W4
Suppose a gas is driven at compressible subsonic speeds by a large pressure drop, p1 to p2.
Describe its behavior on an appropriately labeled Mollier chart for (a) frictionless flow in a
converging nozzle and (b) flow with friction in a long duct.
Solution 9.W4
Problem 9.W5
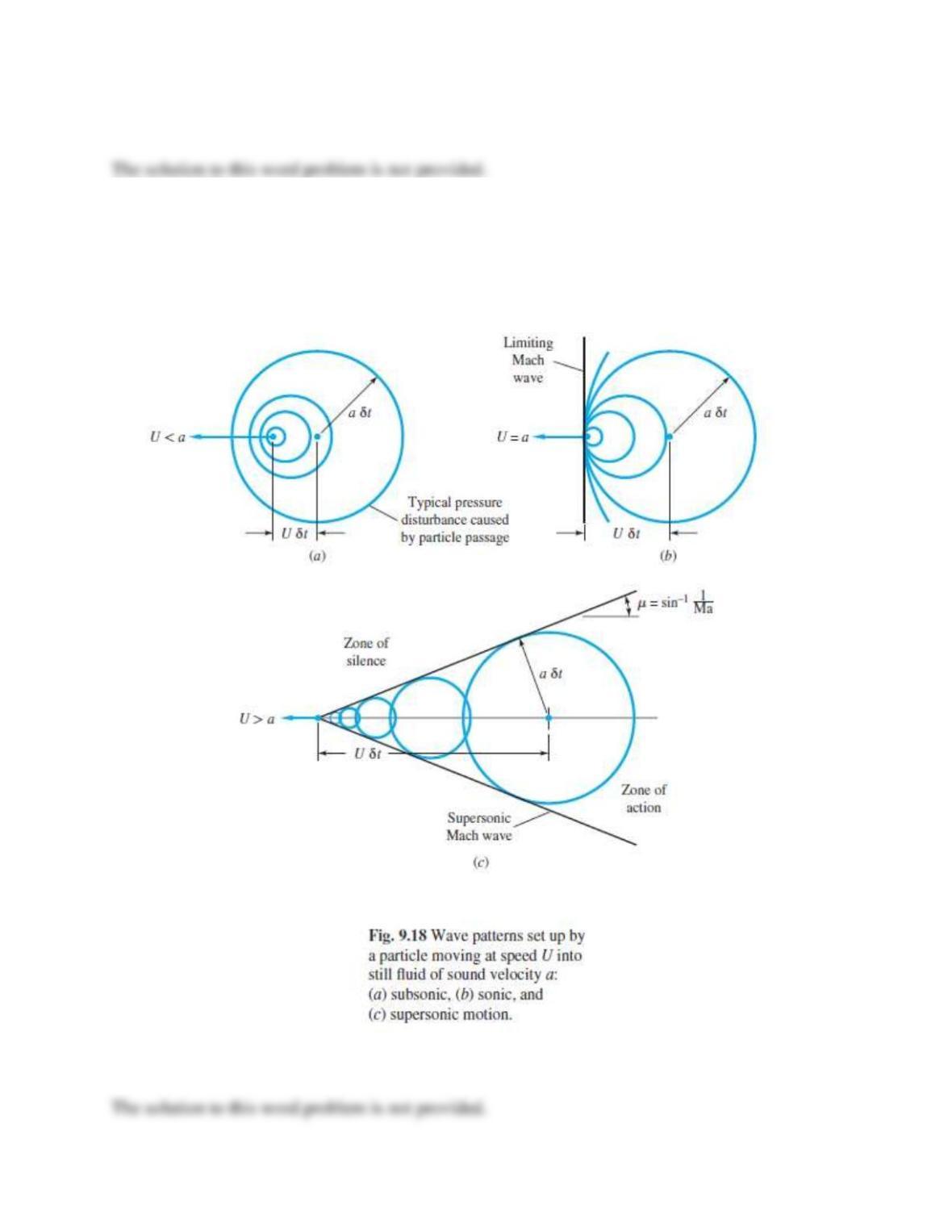
Describe physically what the “speed of sound” represents. What kind of pressure changes occur
in air sound waves during ordinary conversation?
Solution 9.W5
Problem 9.W6
Give a physical description of the phenomenon of choking in a converging-nozzle gas flow.
Could choking happen even if wall friction were not negligible?
Solution 9.W6
The solution to this word problem is not provided.
Problem 9.W7
Shock waves are treated as discontinuities here, but they actually have a very small finite
thickness. After giving it some thought, sketch your idea of the distribution of gas velocity,
pressure, temperature, and entropy through the inside of a shock wave.
Solution 9.W7
The solution to this word problem is not provided.
Problem 9.W8
Describe how an observer, running along a normal shock wave at finite speed V, will see what
appears to be an oblique shock wave. Is there any limit to the running speed?
Solution 9.W8