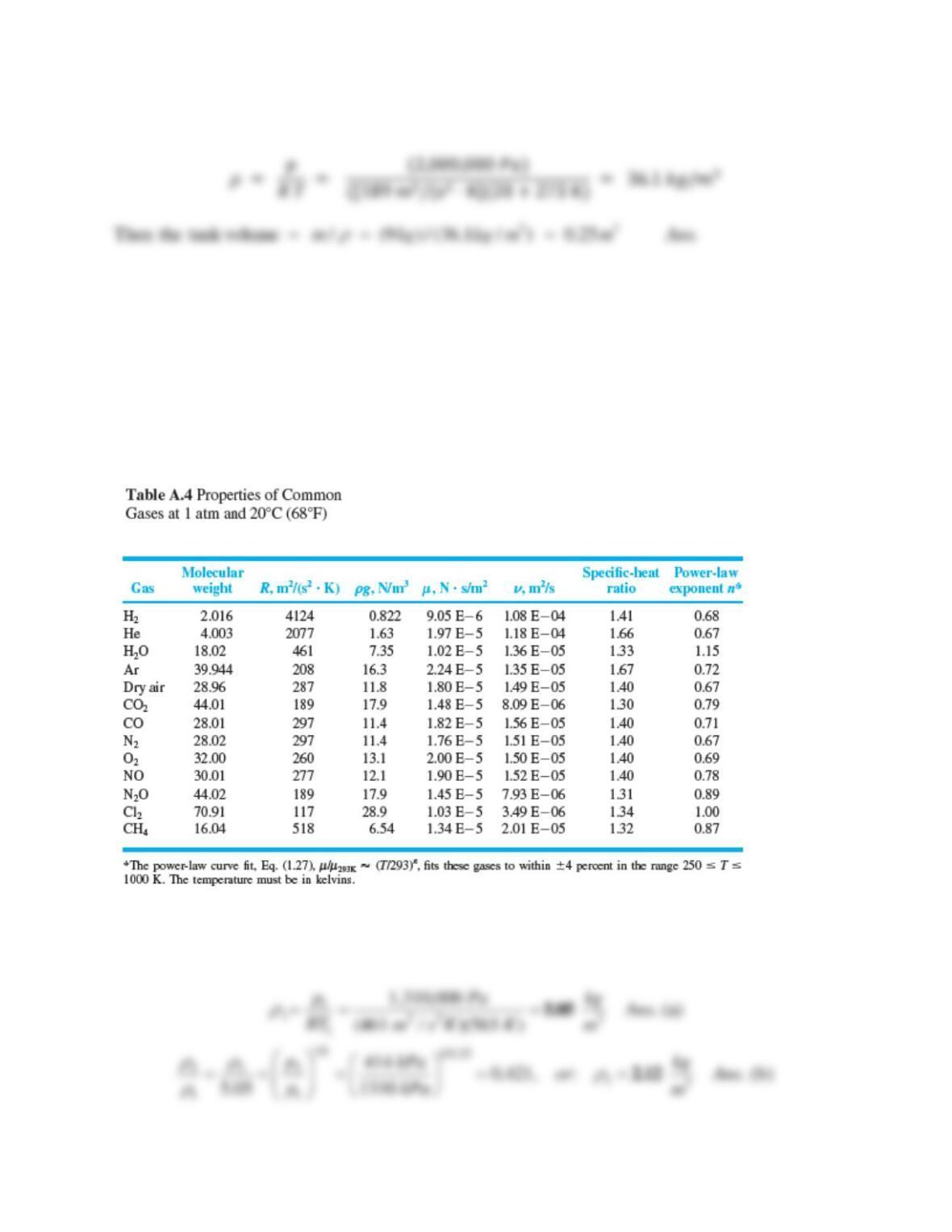
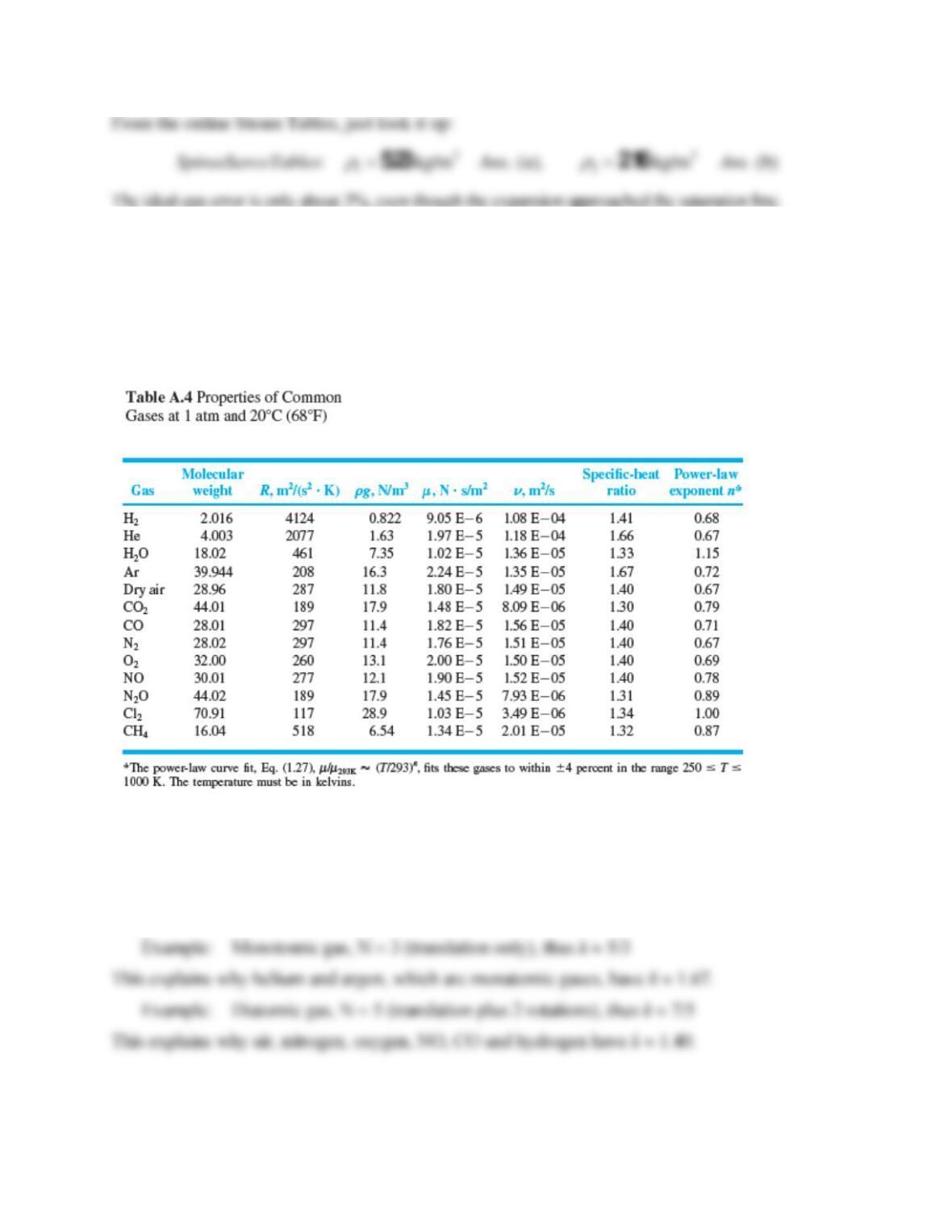
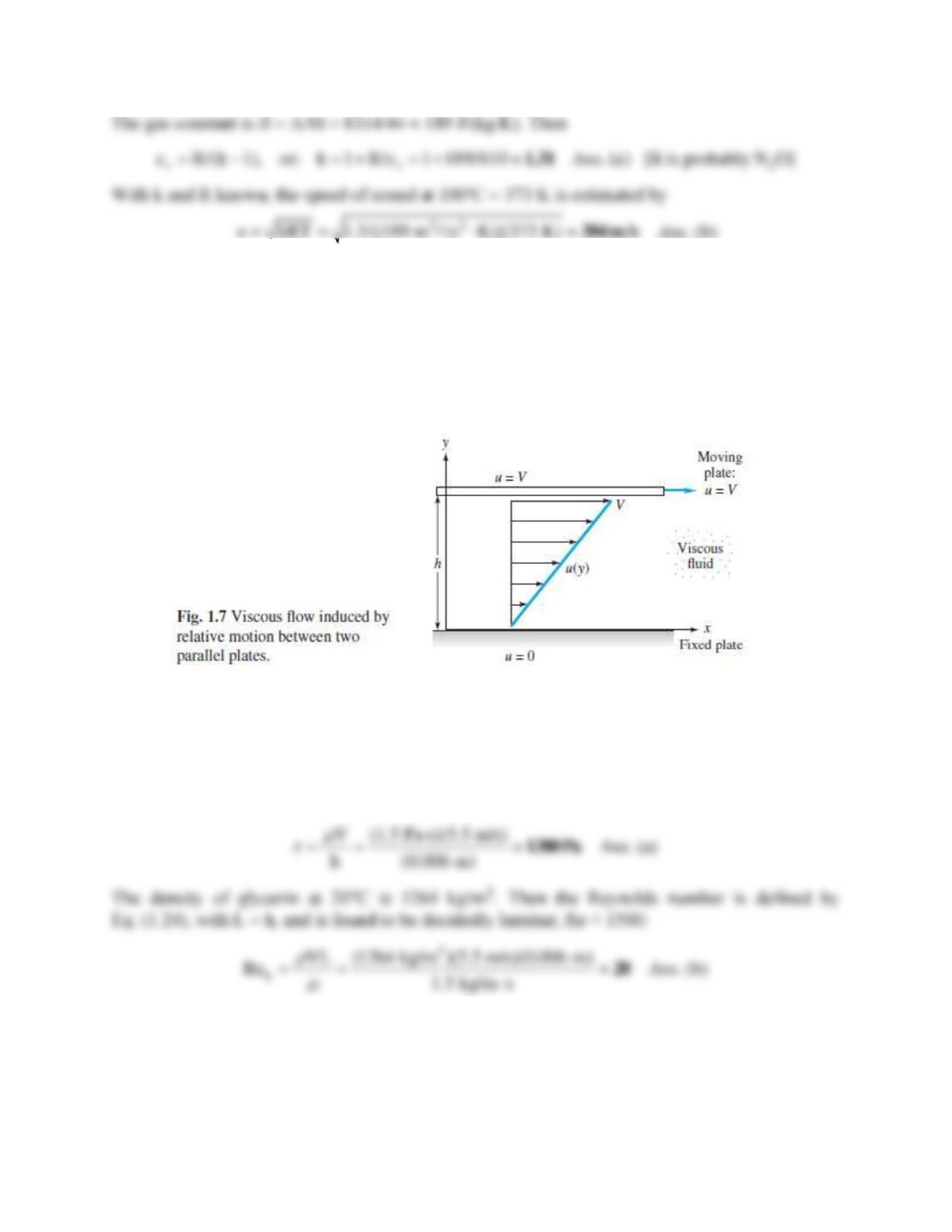
Solution 1.21
Write out the dimensions of each term in the formula:
Problem 1.22
The Ekman number, Ek, arises in geophysical fluid dynamics. It is a dimensionless parameter
combining seawater density
, a characteristic length L, seawater viscosity
, and the Coriolis
frequency
sin
, where is the rotation rate of the earth and
is the latitude angle. Determine
the correct form of Ek if the viscosity is in the numerator.
Solution 1.22
First list the dimensions of the various quantities:
Note that sin
is itself dimensionless, so the Coriolis frequency has the dimensions of .
Problem 1.23
During World War II, Sir Geoffrey Taylor, a British fluid dynamicist, used dimensional analysis to
estimate the energy released by an atomic bomb explosion. He assumed that the energy released, E, was
a function of blast wave radius R, air density
, and time t. Arrange these variables into a single
dimensionless group, which we may term the blast wave number.
Solution 1.23
These variables have the dimensions {E} = {ML2/T2}, {R} = {L}, {
} = {M/L3}, and
-3 -1 -1 -1
{ } {ML } ; { } {L} ; { } {ML T } ; { sin } {T }L
= = = =