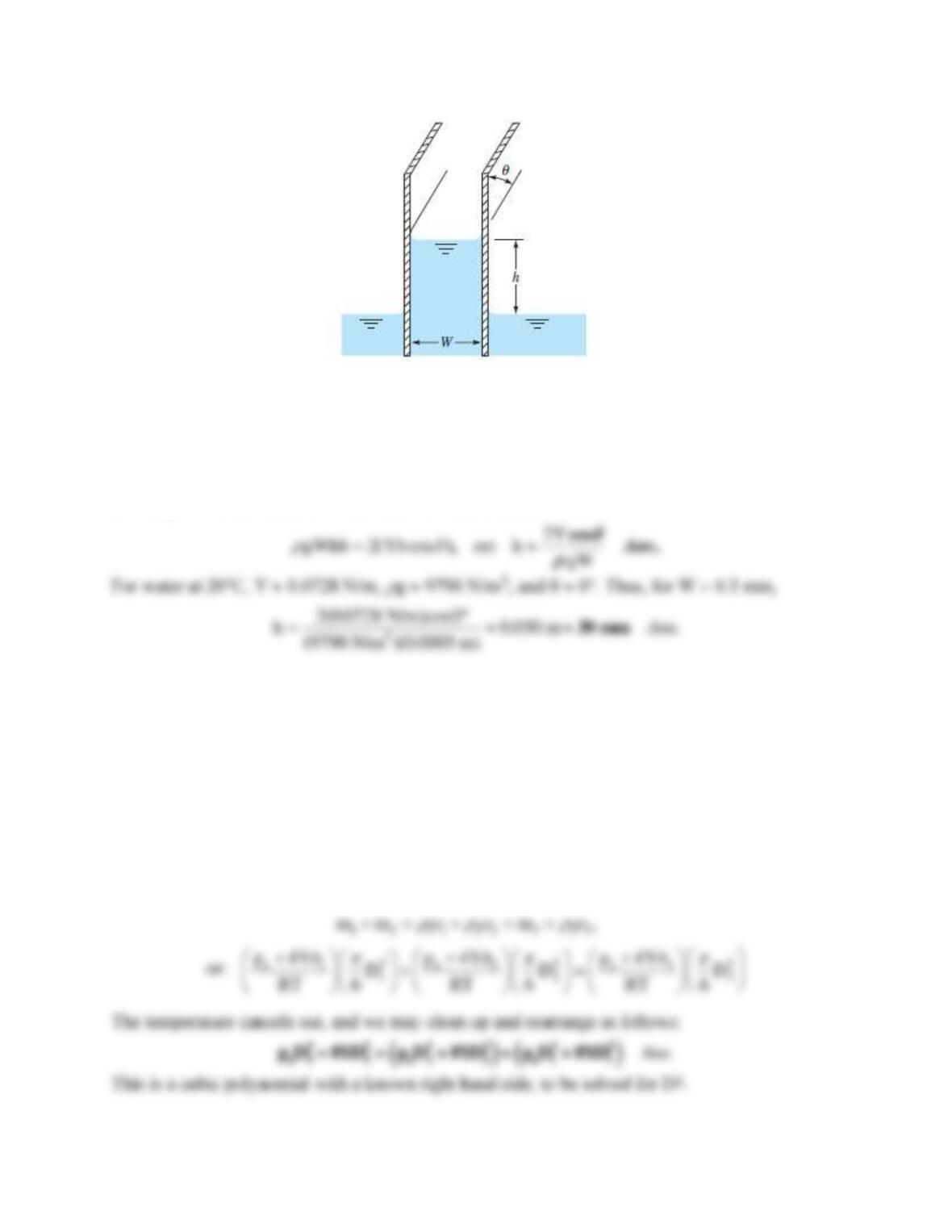
A vertical concentric annulus, with outer radius ro and inner radius ri, is lowered into fluid of
surface tension Y and contact angle
90. Derive an expression for the capillary rise h in the
annular gap if the gap is very narrow.
Solution 1.67
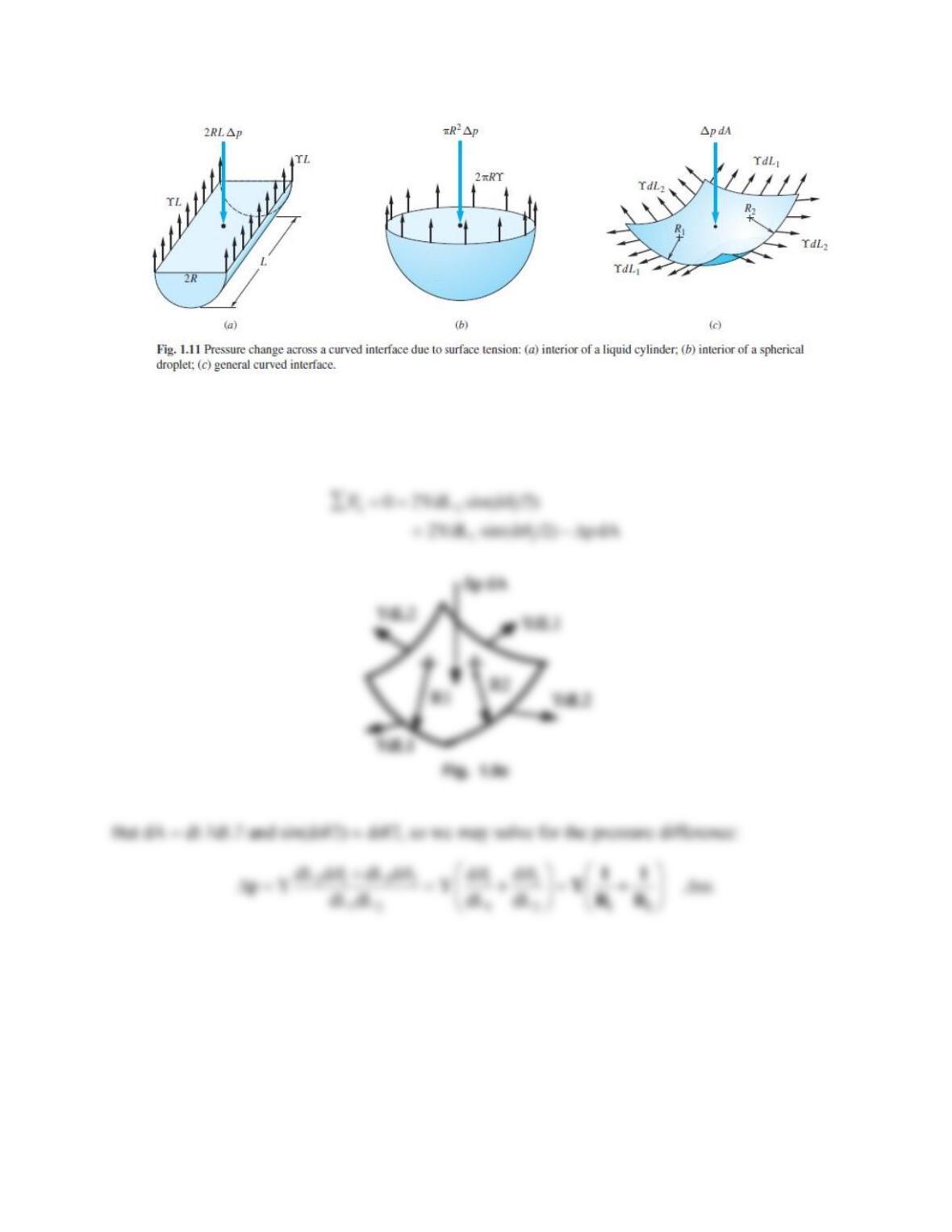
For the figure above, the force balance on the annular fluid is
Problem 1.68*
Make an analysis of the shape
(x) of the water-air interface near a plane wall, as in Fig. P1.68,
assuming that the slope is small, R−1 d2
/dx2. Also assume that the pressure difference across
the interface is balanced by the specific weight and the interface height, p
g
. The boundary
conditions are a wetting contact angle
at x = 0 and a horizontal surface at
= 0 as x → .
What is the maximum height h at the wall?
Figure P1.68
Solution 1.68
This is a two-dimensional surface-tension problem, with single curvature. The surface tension rise is
balanced by the weight of the film. Therefore the differential equation is