c)
Problem 16.6 .
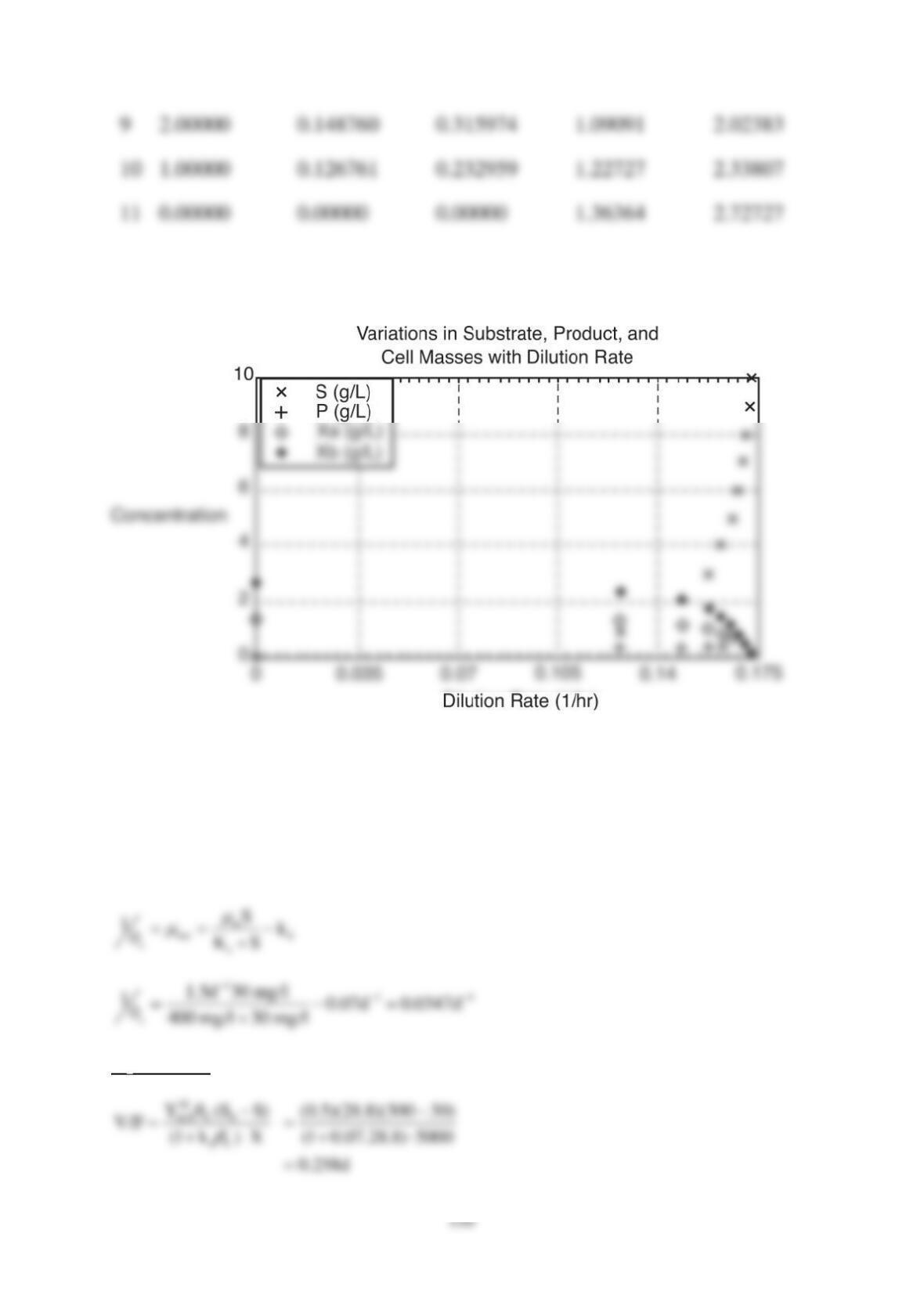
F = 6 m3/h , COD0 = S0 = 1000 mg/l , CODe = S = 30 mg/l , k = 5 d-1 ,
KS = 125 mg/l , Y = 0.35 gX/gS , b = 0.03 d-1 , SVI = 125 ml/g , V = 50 m3
r = 0.362
Sludge Production rate
PrX = F(1+r)X –r F Xr = 6(1.362)(2.887) – 0.362(6) (10) = 1.872 kgX/h = 44.93 kgX/d
b. r = 0.3 , S = 30 mg/L , cm = 0.655 d , c = 3.24 d
Problem 16.7.
F = 10 m3/h , S0 = 2000 mg/L , k = 6 d-1 , Ks = 150 mg/L , Y = 0.4 gX/gS
b = 0.02 d-1 , SVI = 100 ml/g , r= 0.4 , c = 4 cm