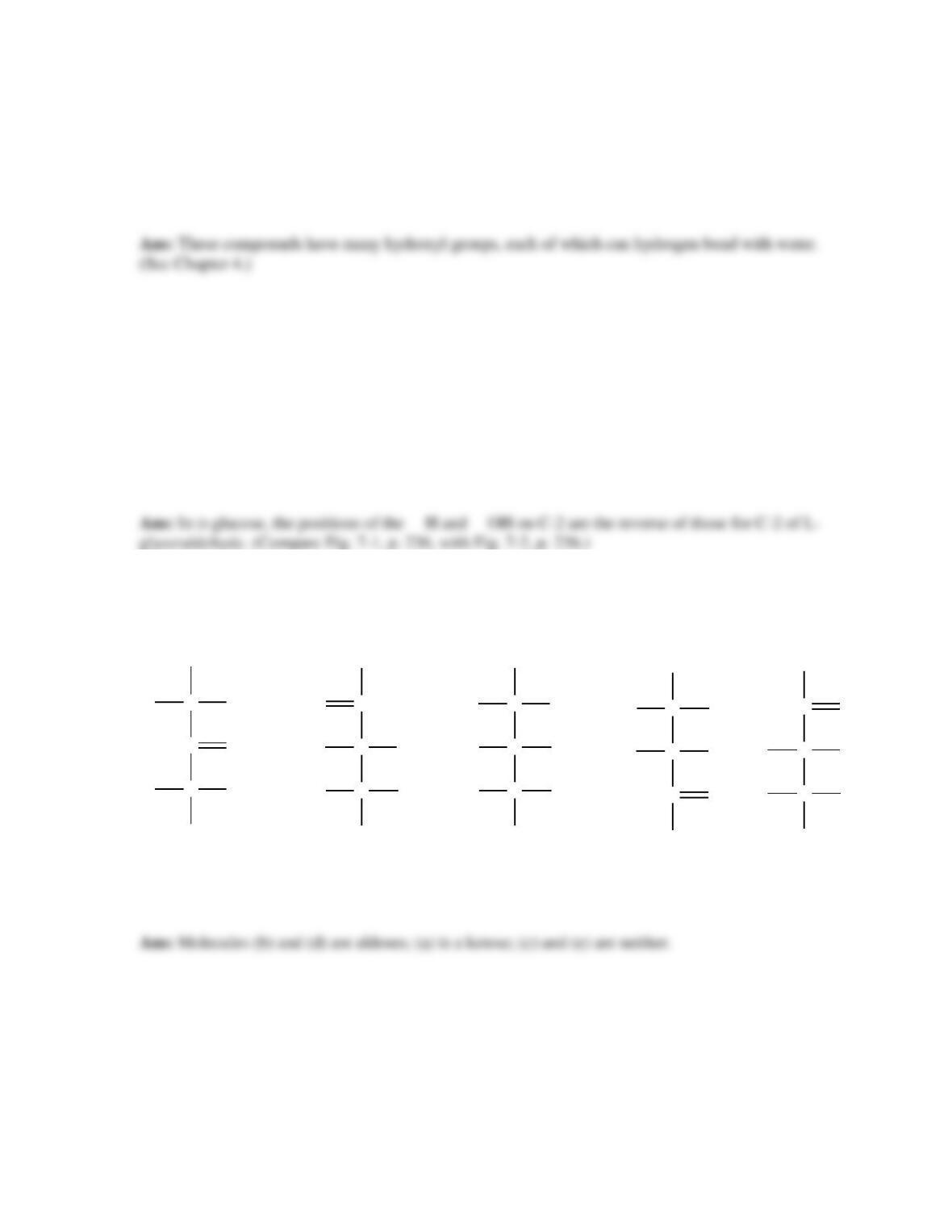
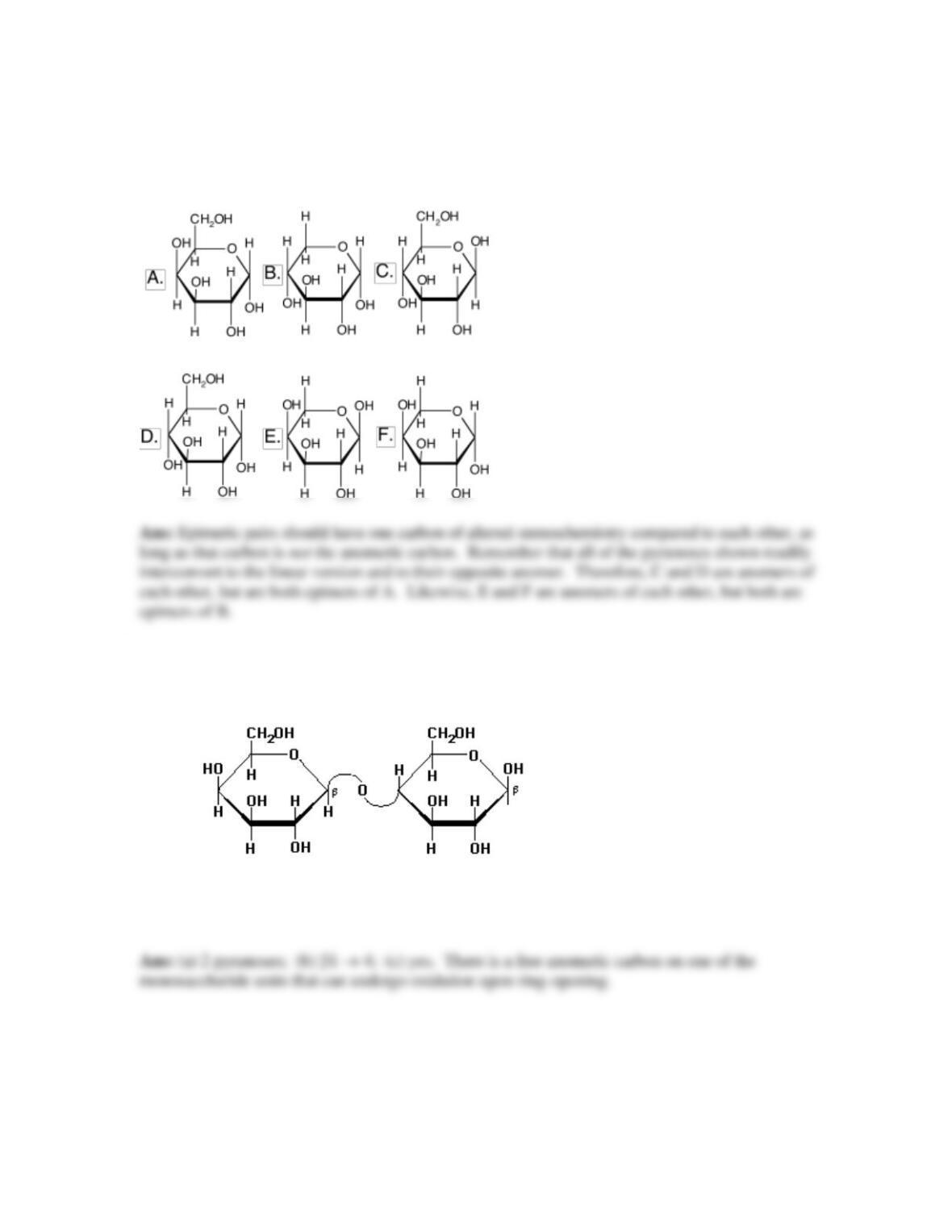
20. Glycoconjugates: proteoglycans, glycoproteins, and glycolipids
The basic structure of a proteoglycan consists of a core protein and a:
A) glycolipid.
B) glycosaminoglycan.
C) lectin.
D) lipopolysaccharide.
E) peptidoglycan.
21. Glycoconjugates: proteoglycans, glycoproteins, and glycolipids
Which of the following statements about heparan sulfate is not true?
A) Sulfation of heparan sulfate to form NS domains is important for its role as an anti-coagulant.
B) Heparan sulfate can promote protein-protein interactions via the NS domains.
C) The secondary structure of heparan sulfate is completely random.
D) The NA domains of heparan sulfate contain no sulfation.
E) The core repeating structure of heparan sulfate is made up of alternating GlcNAc and GlcA.
22. Glycoconjugates: proteoglycans, glycoproteins, and glycolipids
In glycoproteins, the carbohydrate moiety is always attached through the amino acid residues:
A) asparagine, serine, or threonine.
B) aspartate or glutamate.
C) glutamine or arginine.
D) glycine, alanine, or aspartate.
E) tryptophan, aspartate, or cysteine.
23. Glycoconjugates: proteoglycans, glycoproteins, and glycolipids
Which of the following is a dominant feature of the outer membrane of the cell wall of gram negative
bacteria?
A) Amylose
B) Cellulose
C) Glycoproteins
D) Lipopolysaccharides
E) Lipoproteins
24. Carbohydrates as informational molecules: the sugar code
The biochemical property of lectins that is the basis for most of their biological effects is their ability
to bind to:
A) amphipathic molecules.
B) hydrophobic molecules.
C) specific lipids.
D) specific oligosaccharides.
E) specific peptides.