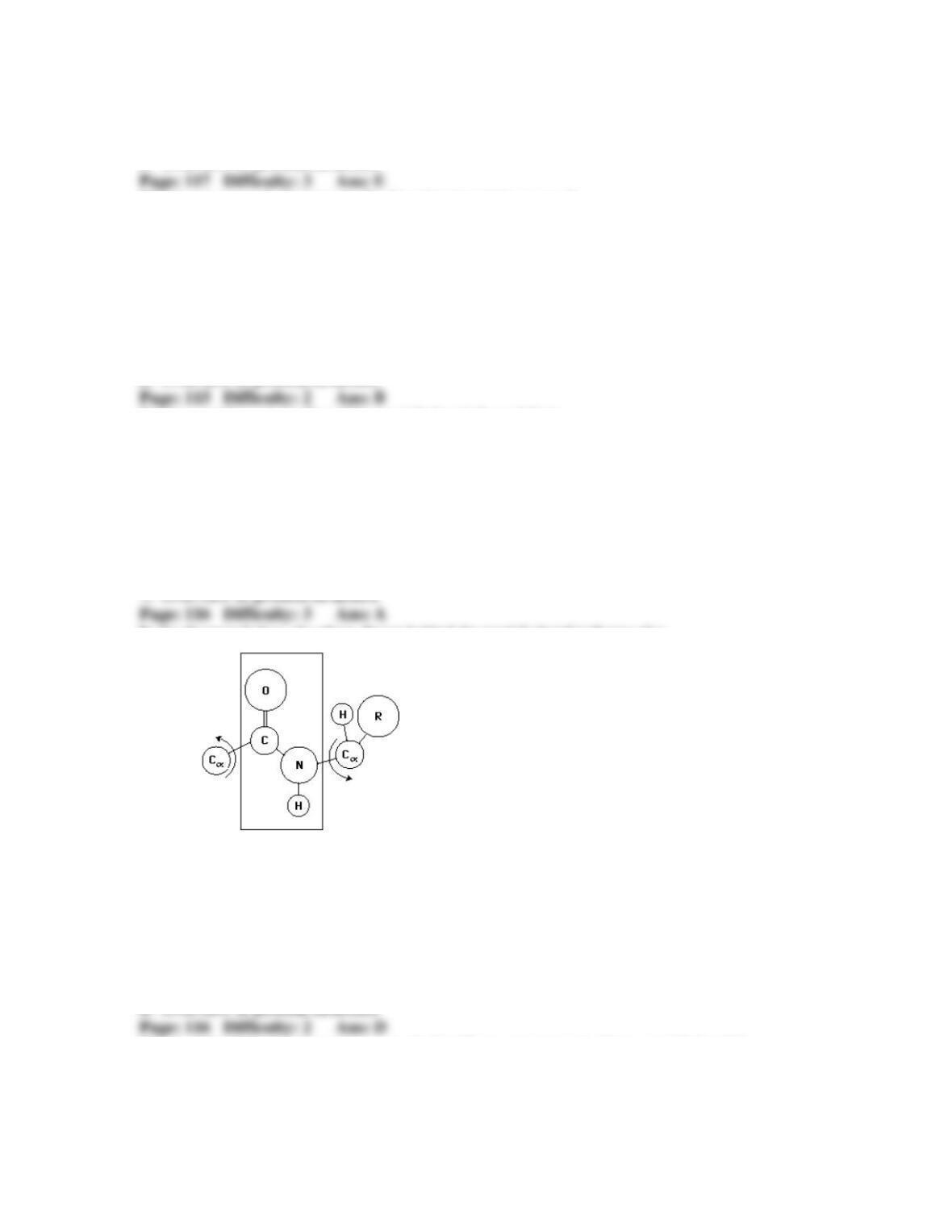
31. Protein denaturation and folding
Which of the following is least likely to result in protein denaturation?
A) Altering net charge by changing pH
B) Changing the salt concentration
C) Disruption of weak interactions by boiling
D) Exposure to detergents
E) Mixing with organic solvents such as acetone
32. Protein denaturation and folding
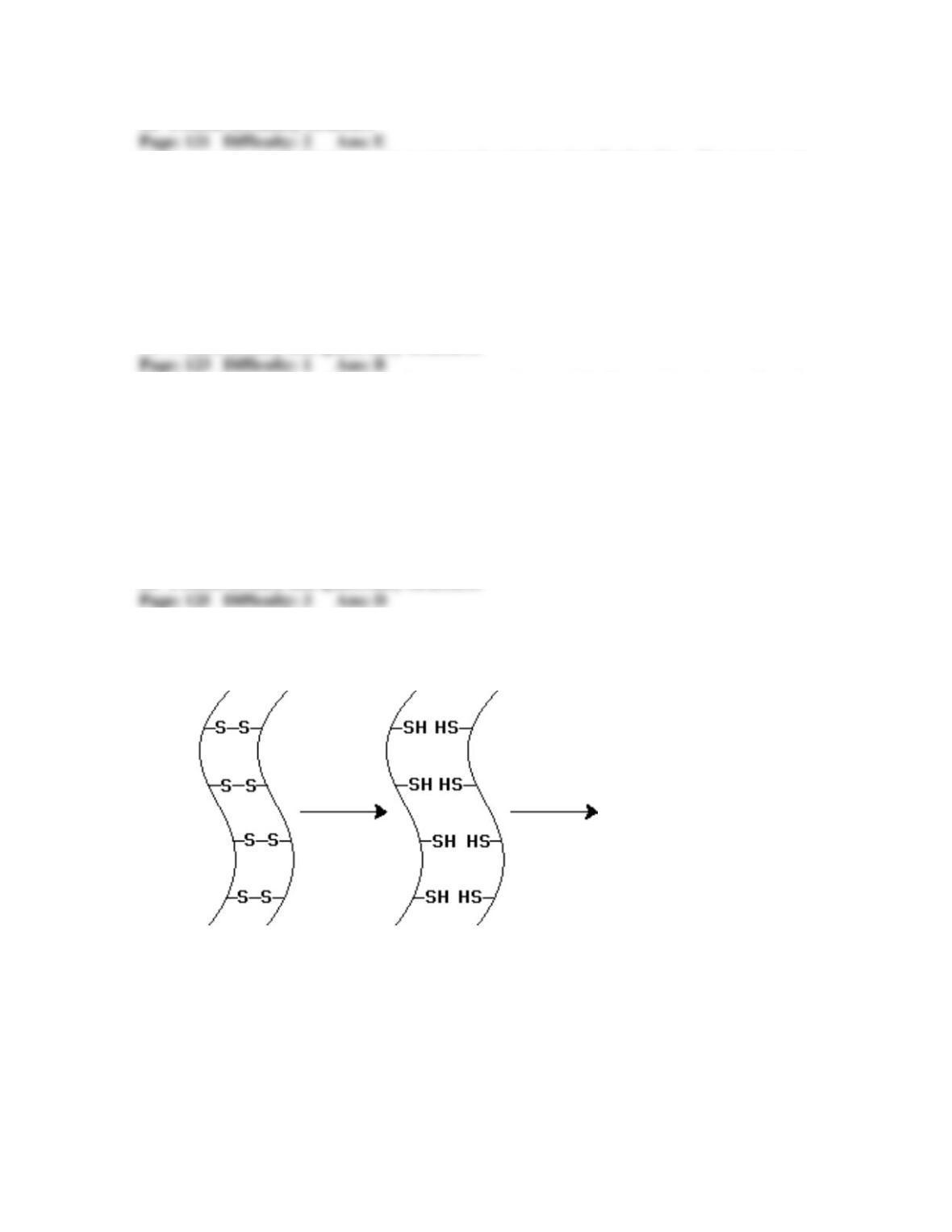
Experiments on denaturation and renaturation after the reduction and reoxidation of the —S—S—
bonds in the enzyme ribonuclease (RNase) have shown that:
A) folding of denatured RNase into the native, active conformation, requires the input of energy in
the form of heat.
B) native ribonuclease does not have a unique secondary and tertiary structure.
C) the completely unfolded enzyme, with all —S—S— bonds broken, is still enzymatically active.
D) the enzyme, dissolved in water, is thermodynamically stable relative to the mixture of amino
acids whose residues are contained in RNase.
E) the primary sequence of RNase is sufficient to determine its specific secondary and tertiary
structure.
33. Protein denaturation and folding
Which of the following statements concerning the process of spontaneous folding of proteins is false?
A) It may be an essentially random process.
B) It may be defective in some human diseases.
C) It may involve a gradually decreasing range of conformational species.
D) It may involve initial formation of a highly compact state.
E) It may involve initial formation of local secondary structure.
34. Protein denaturation and folding
Protein S will fold into its native conformation only when protein Q is also present in the solution.
However, protein Q can fold into its native conformation without protein S. Protein Q, therefore,
may function as a ____________ for protein S.
A) proteasome
B) molecular chaperone
C) protein precursor
D) structural motif
E) supersecondary structural unit
35. Protein denaturation and folding
Which of the following is not known to be involved in the process of assisted folding of proteins?