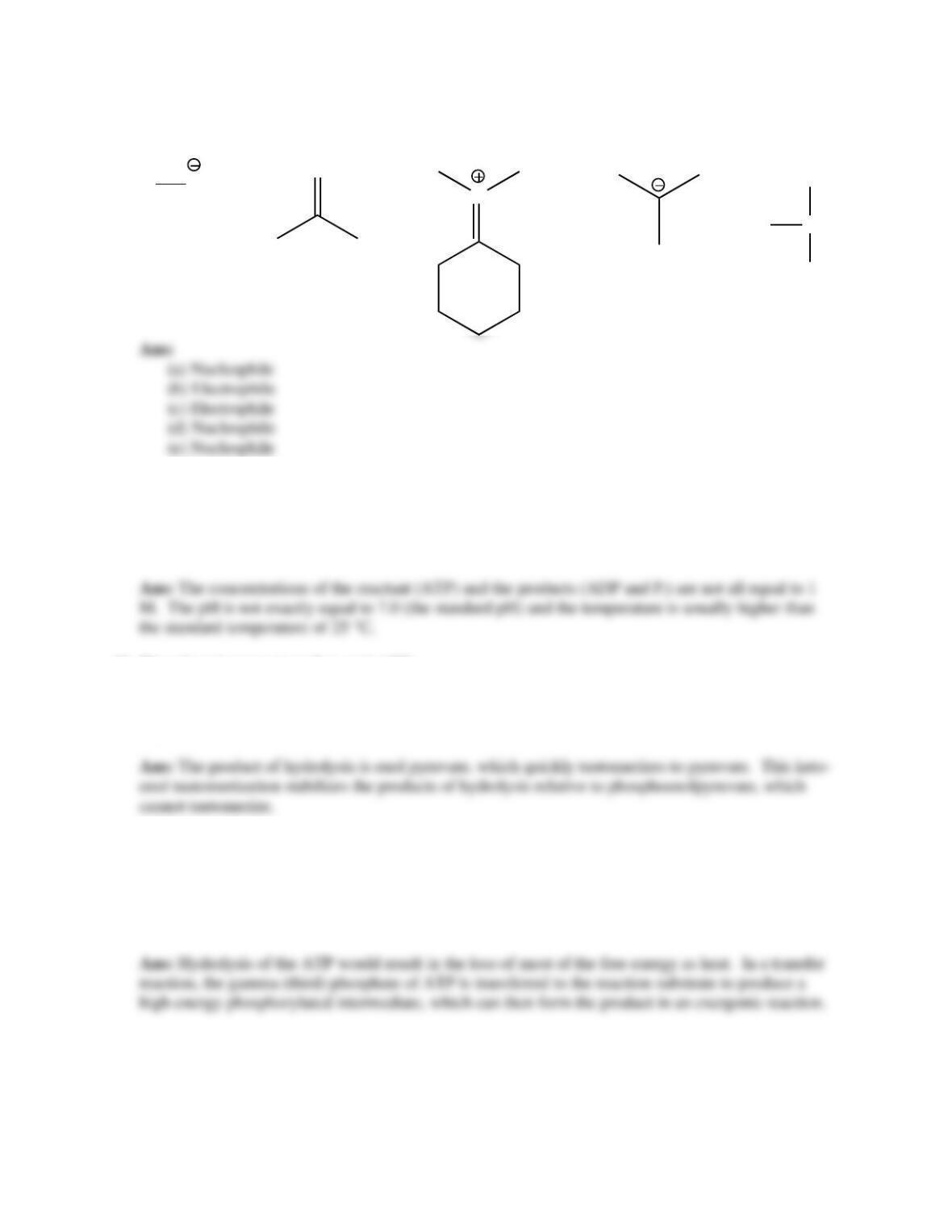
8. Bioenergetics and thermodynamics
For the following reaction, G'° = +29.7 kJ/mol.
L-Malate + NAD+ → oxaloacetate + NADH + H+
The reaction as written:
A) can never occur in a cell.
B) can occur in a cell only if it is coupled to another reaction for which G'° is positive.
C) can occur only in a cell in which NADH is converted to NAD+ by electron transport.
D) cannot occur because of its large activation energy.
E) may occur in cells at some concentrations of substrate and product.
9. Bioenergetics and thermodynamics
For the reaction A → B, the Keq' is 104. If a reaction mixture originally contains 1 mmol of A and no
B, which one of the following must be true?
A) At equilibrium, there will be far more B than A.
B) The rate of the reaction is very slow.
C) The reaction requires coupling to an exergonic reaction in order to proceed.
D) The reaction will proceed toward B at a very high rate.
E) G'° for the reaction will be large and positive.
10. Bioenergetics and thermodynamics
For the reaction A → B, the Keq' is 10-6. If a reaction mixture originally contains 1 mmol of A and 1
mmol of B, which one of the following must be true?
A) At equilibrium, there will be still be equal levels of A and B.
B) The rate of the reaction is very slow.
C) At equilibrium, the amount of A will greatly exceed the amount of B.
D) The reaction will proceed toward B at a very high rate.
E) G'° for the reaction will be large and positive.
11. Bioenergetics and thermodynamics
In glycolysis, fructose 1,6-bisphosphate is converted to two products with a standard free-energy
change (G'°) of 23.8 kJ/mol. Under what conditions encountered in a normal cell will the free-
energy change (G) be negative, enabling the reaction to proceed spontaneously to the right?
A) Under standard conditions, enough energy is released to drive the reaction to the right.
B) The reaction will not go to the right spontaneously under any conditions because the G'° is
positive.
C) The reaction will proceed spontaneously to the right if there is a high concentration of products
relative to the concentration of fructose 1,6-bisphosphate.
D) The reaction will proceed spontaneously to the right if there is a high concentration of fructose
1,6-bisphosphate relative to the concentration of products.
E) None of the above conditions is sufficient.