8-10
thetaI 1 1 1 1
CpI 40 40 40 40
Ea 2.5E+04 2.5E+04 2.5E+04 2.5E+04
Kc 66.01082 0.8247864 66.01082 31.551036
ka 0.046809 0.046809 11.205249 0.1177827
xe 0.8024634 0.3122841 0.8024634 0.7374305
sumcp 80 80 80 80
Ca 0.1060606 0.0137198 0.1060606 0.0137198
Cc 0 0 0.0724316 0.0355685
ra -5.265E-04 -0.0143957 -1.745E-05 -1.745E-05
ODE Report (RKF45)
Differential equations as entered by the user
[1] d(Ta)/d(W) = Uarho*(T-Ta)/(Mc*Cpmc)
[2] d(y)/d(W) = -alpha/2*(T/To)/y
[3] d(T)/d(W) = (Uarho*(Ta-T)+(-ra)*(-Hr))/(Fao*sumcp)
Explicit equations as entered by the user
[1] alpha = .0002
[2] To = 350
[3] Uarho = 0.5
[4] Mc = 200
[5] Cpmc = 18
[8] thetaI = 1
[9] CpI = 40
[10] CpA = 20
[11] thetaB = 1
[12] CpB = 20
[13] Cto = 0.3
[14] Ea = 25000
[15] Kc = 1000*(exp(Hr/1.987*(1/303-1/T)))
[16] ka = .004*exp(Ea/1.987*(1/310-1/T))
[20] sumcp = (thetaI*CpI+CpA+thetaB*CpB)
[21] Ca = Cao*(1-X)*y*To/T
[22] Cb = Cao*(1-X)*y*To/T
[23] Cc = Cao*2*X*y*To/T
P8-2 (n)
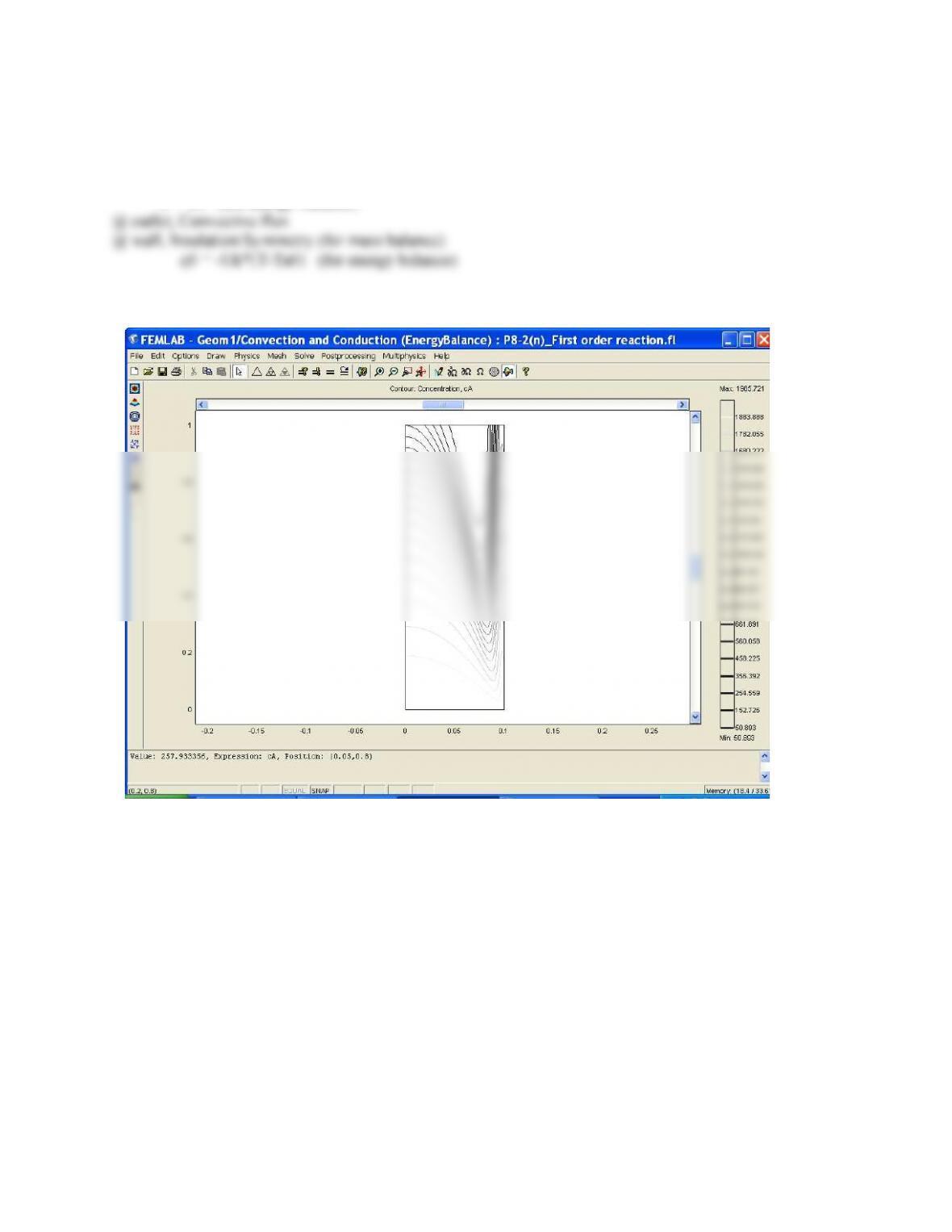
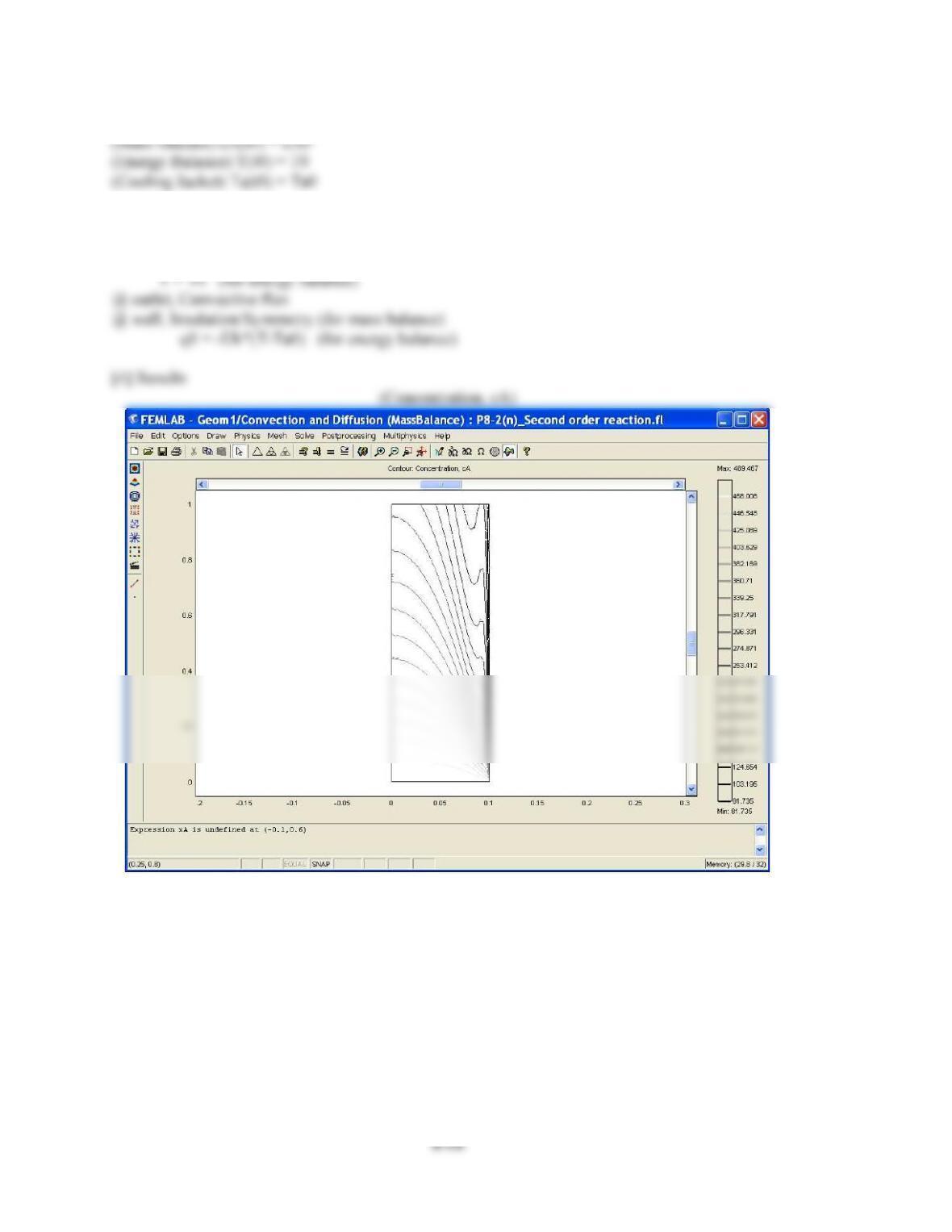
(1) The concentration of A near the wall is lower than in the center because the velocity profile is
parabolic. This means near the walls the velocity is much lower and therefore the time space near