8-31
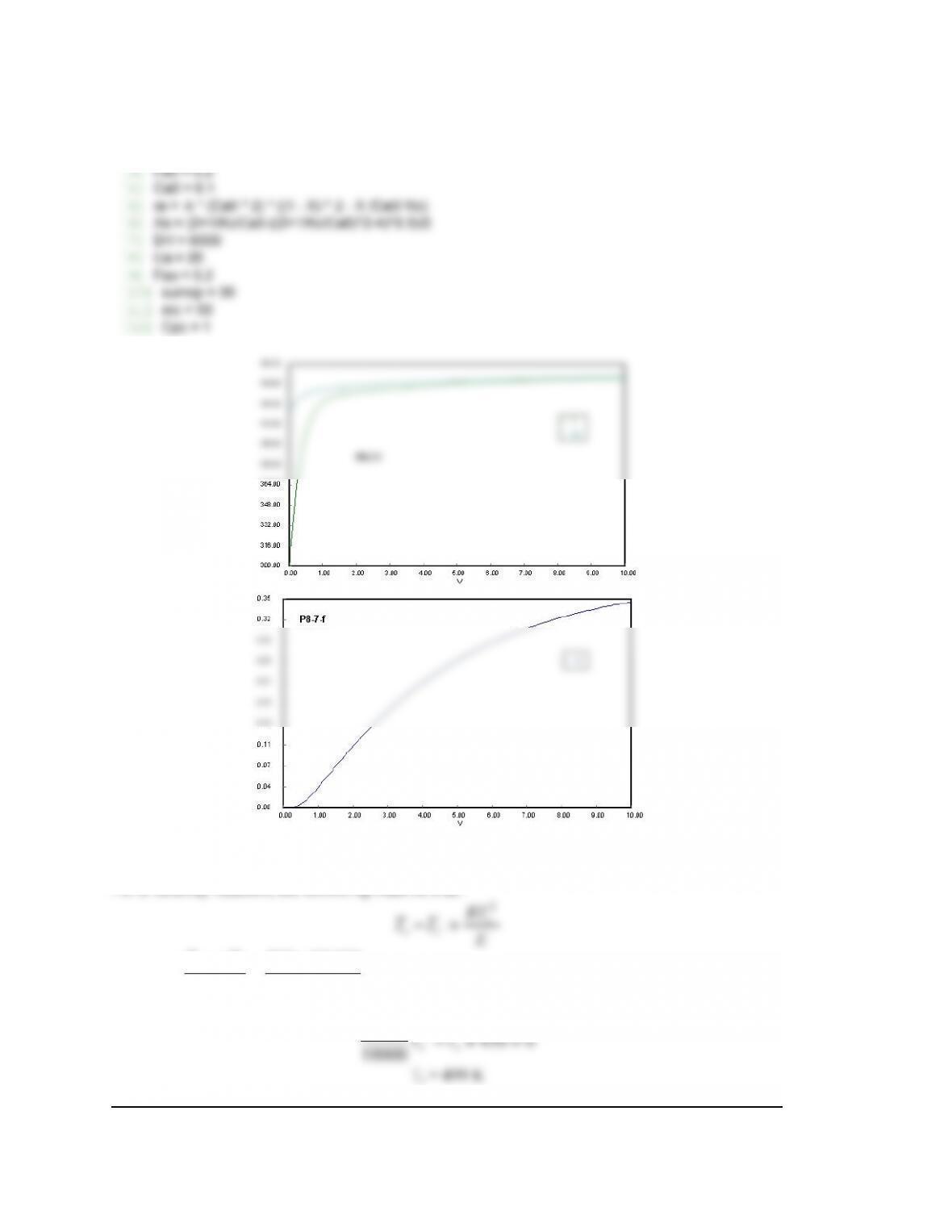
P8-7 (d)
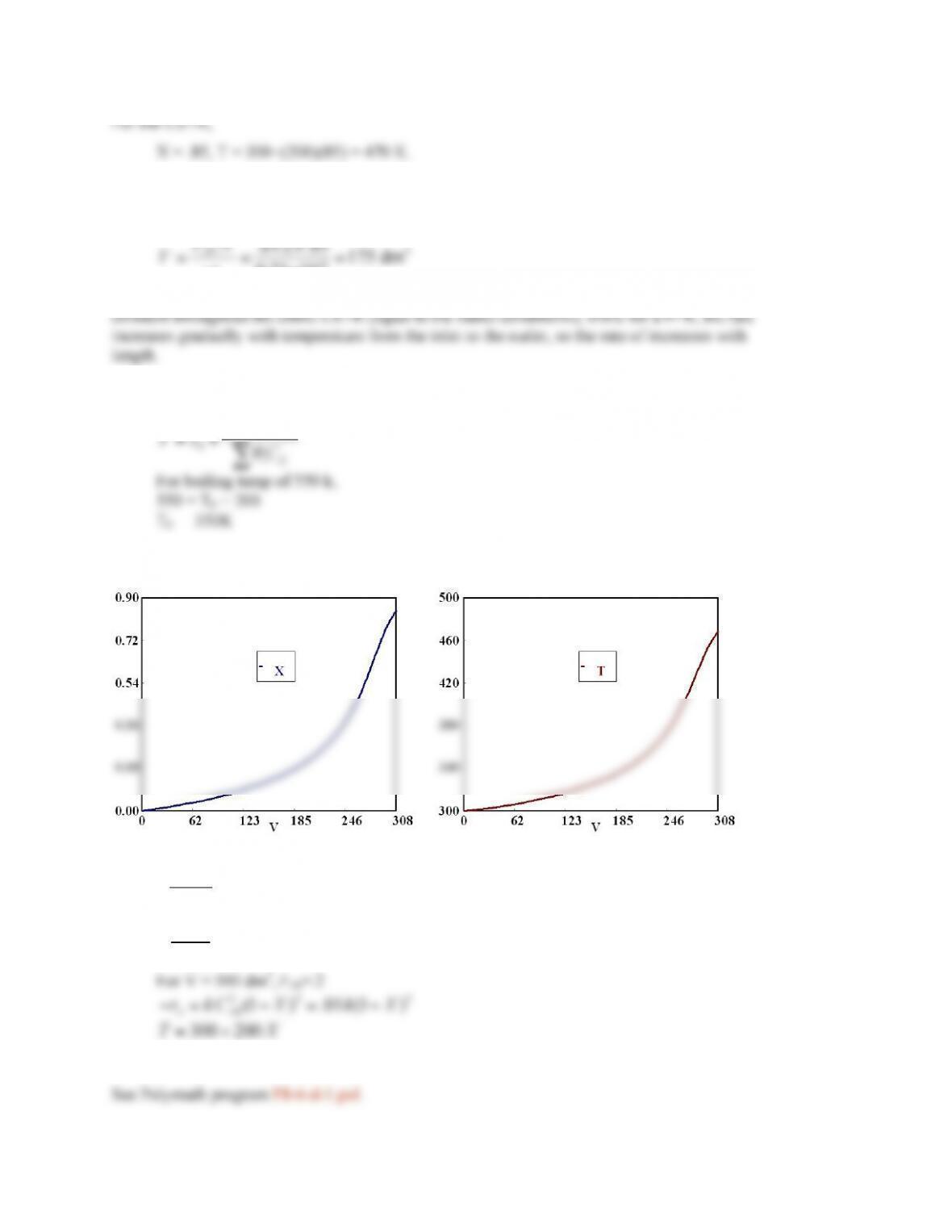
For counter-current flow,
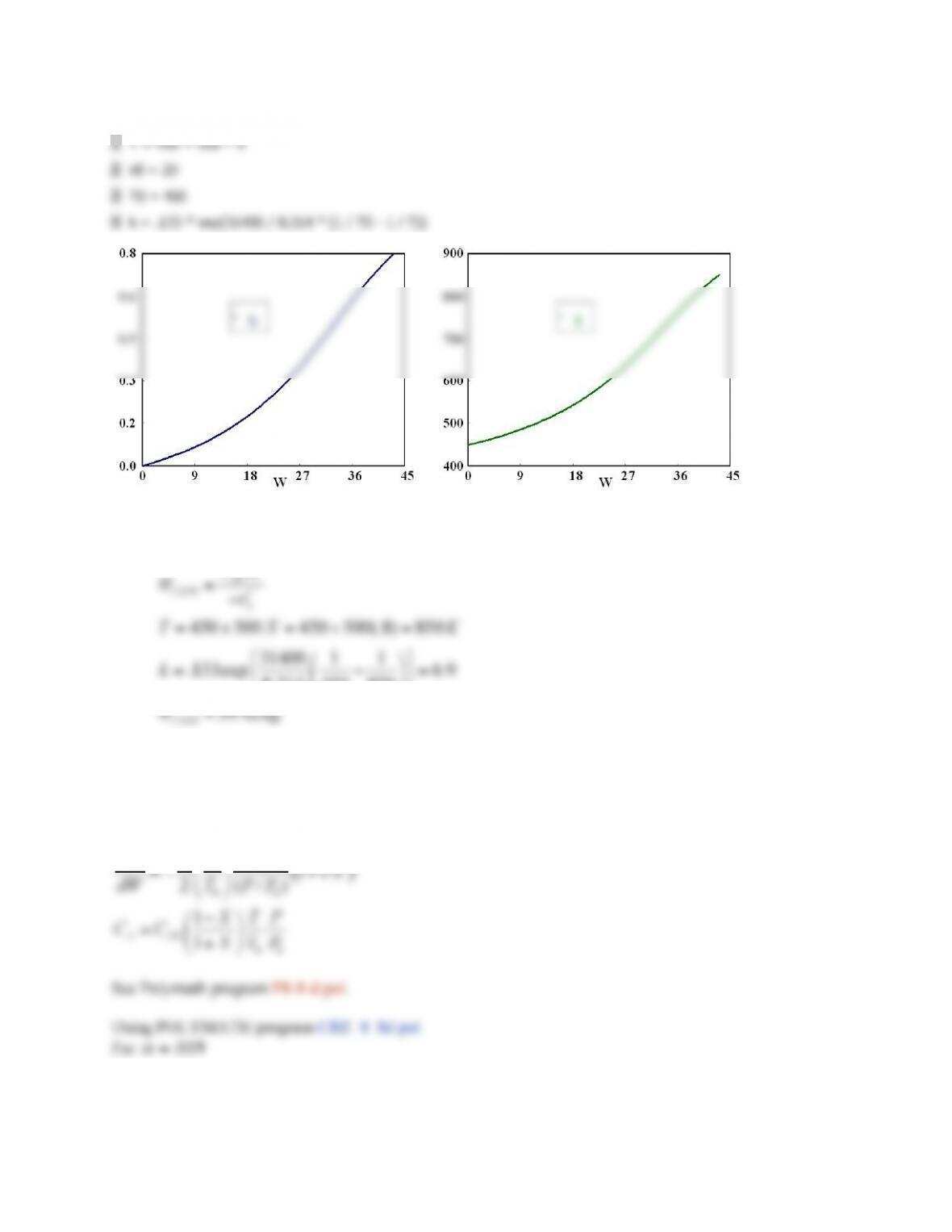
See Polymath program P8-7-d.pol.
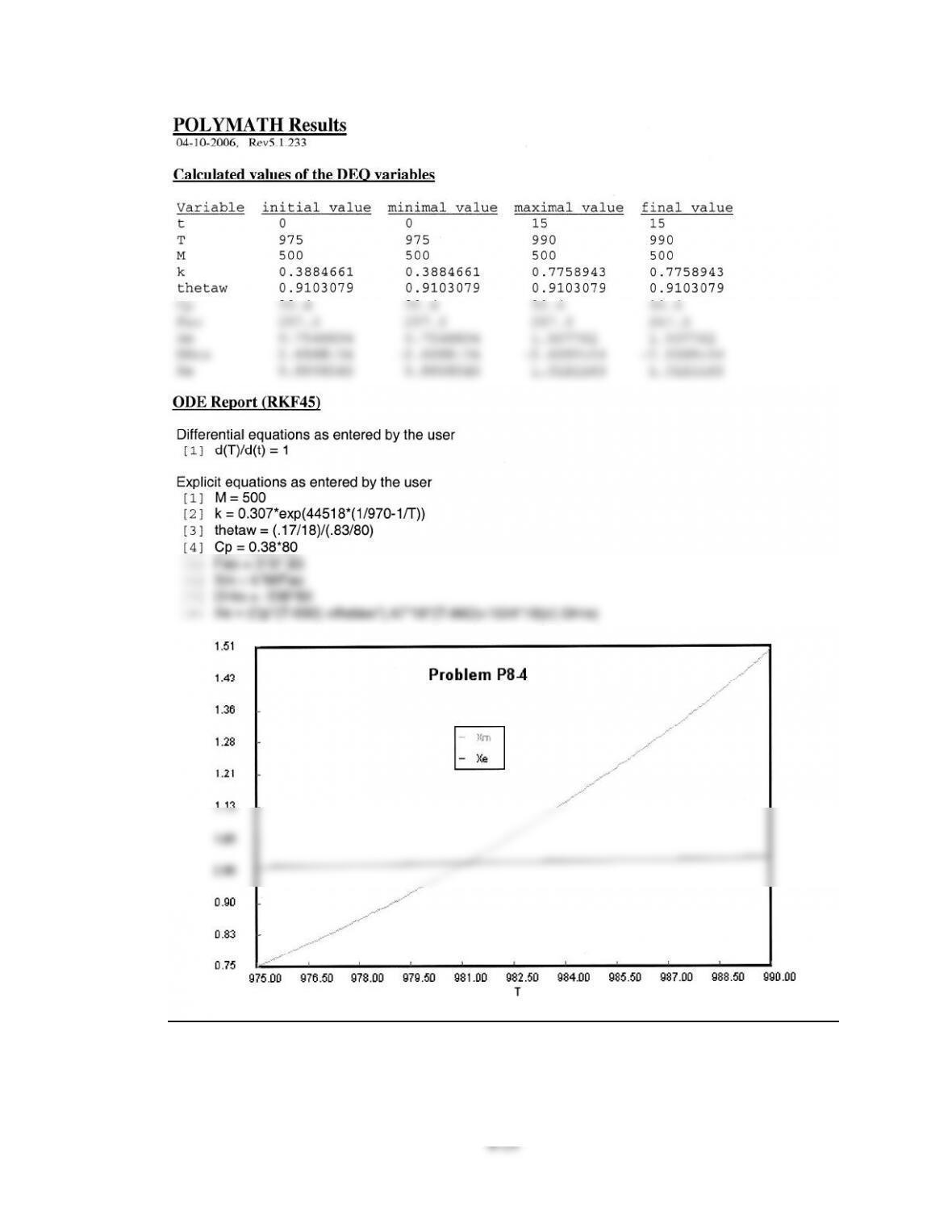
Calculated values of the DEQ variables
Variable initial value minimal value maximal value final value
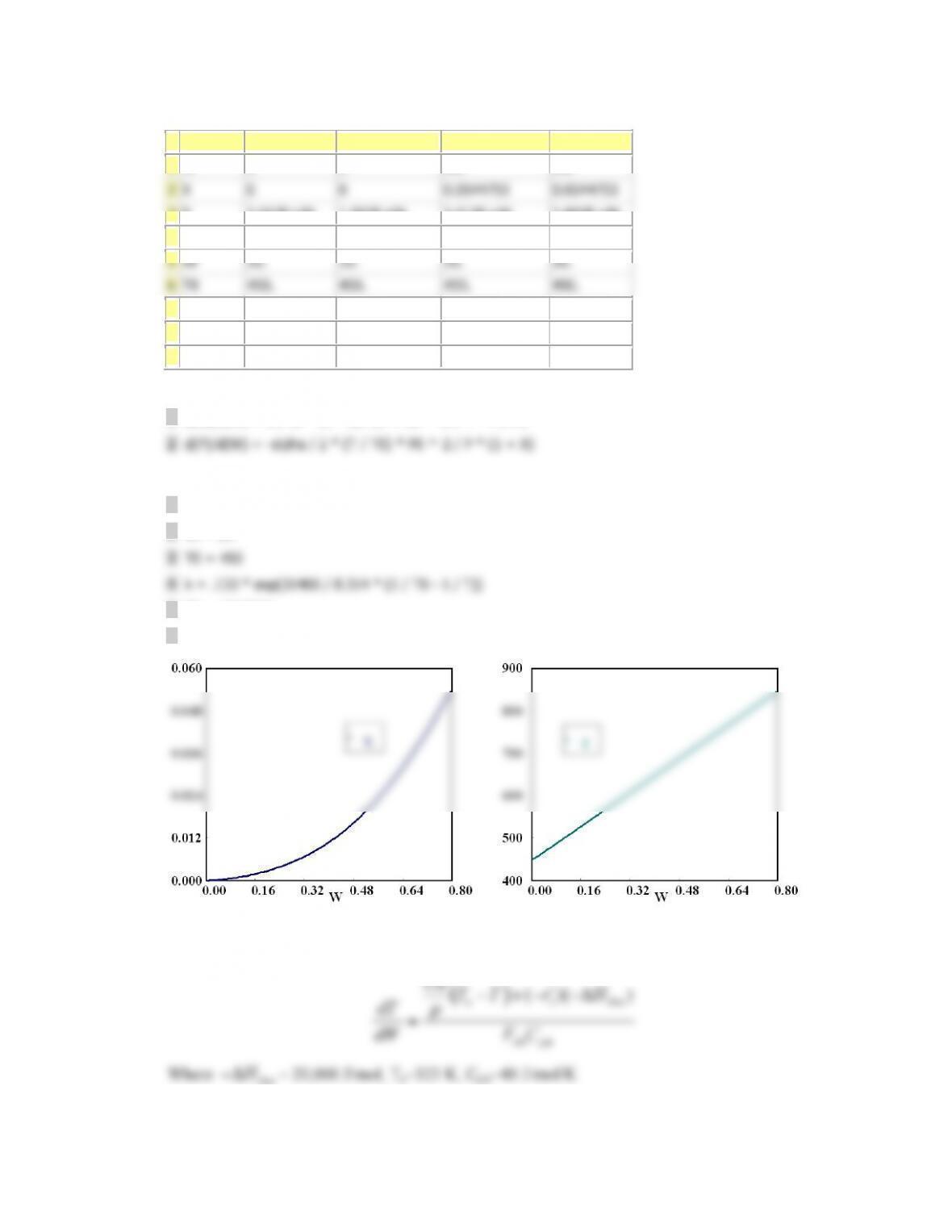
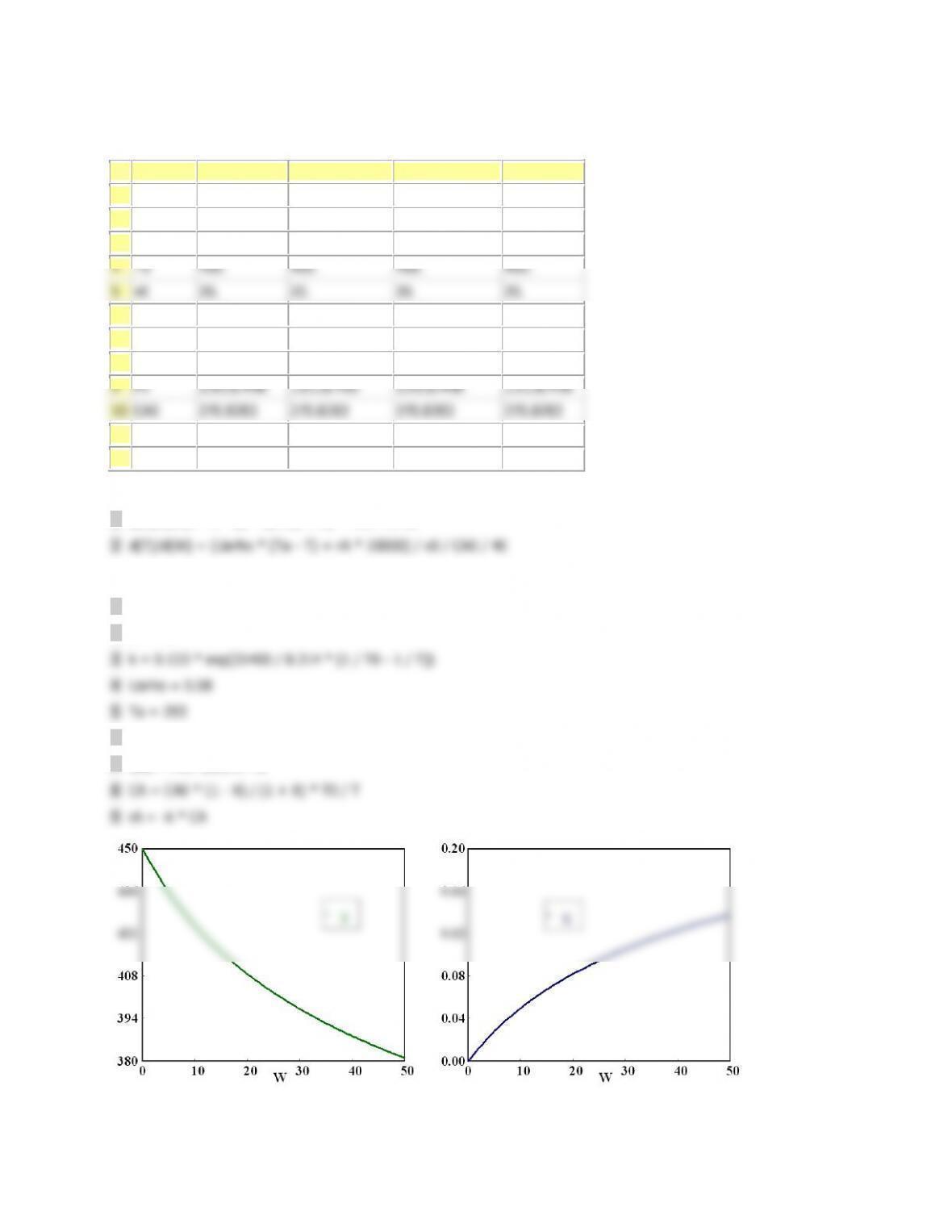
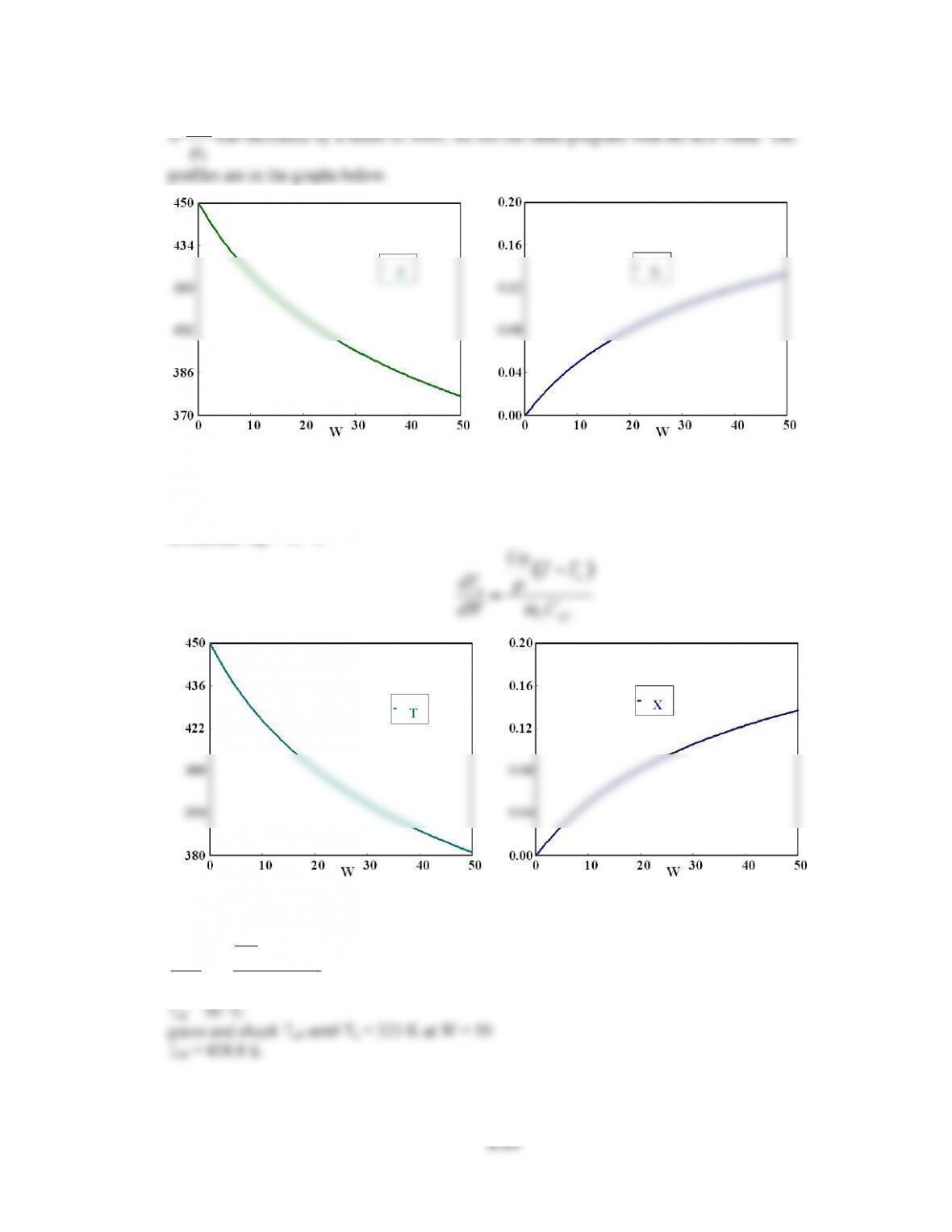
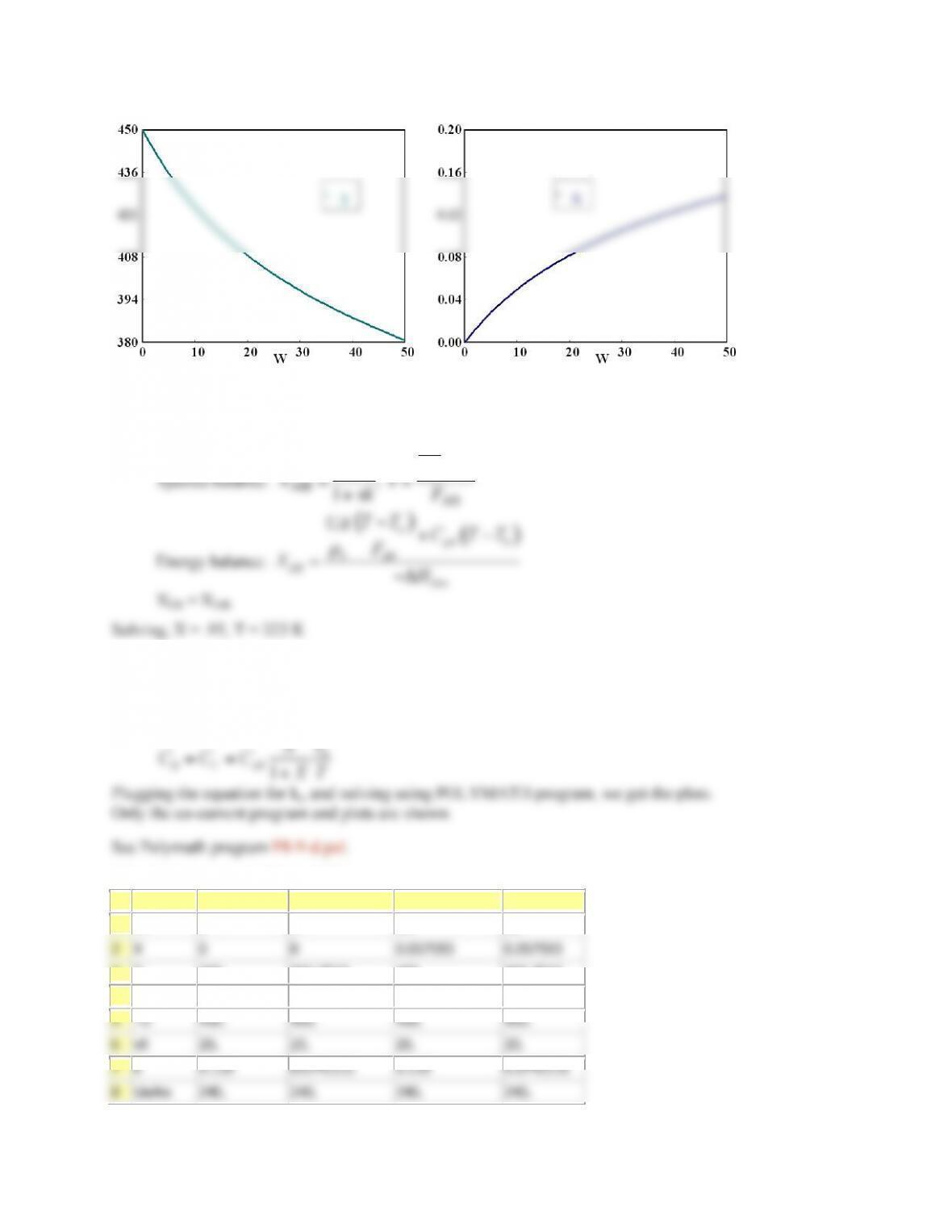
V 0 0 10 10
X 0 0 0.3647241 0.3647241
T 300 300 463.44558 450.37724
Ta 440.71 440.71 457.98124 450.00189
Fa0 0.2 0.2 0.2 0.2
ra -1.0E-04 -0.0256436 -1.0E-04 -9.963E-04
Xe 0.8298116 0.3488462 0.8298116 0.381006
DH -6000 -6000 -6000 -6000
sumcp 30 30 30 30
mc 50 50 50 50
ODE Report (RKF45)
Differential equations as entered by the user
[1] d(X)/d(V) = -ra / Fa0
[2] d(T)/d(V) = (Ua*(Ta-T)+(ra)*DH)/(Fao*sumcp)
Explicit equations as entered by the user
[1] k = .01 * exp((10000 / 1.987) * (1 / 300 - 1 / T))
[2] Kc = 10 * exp(-6000 / 1.987 * (1 / 450 - 1 / T))
[3] Fa0 = 0.2
[4] Ca0 = 0.1
[5] ra = -k * (Ca0 ^ 2) * ((1 - X) ^ 2 - X /Ca0/ Kc)
[11] mc = 50
[12] Cpc = 1