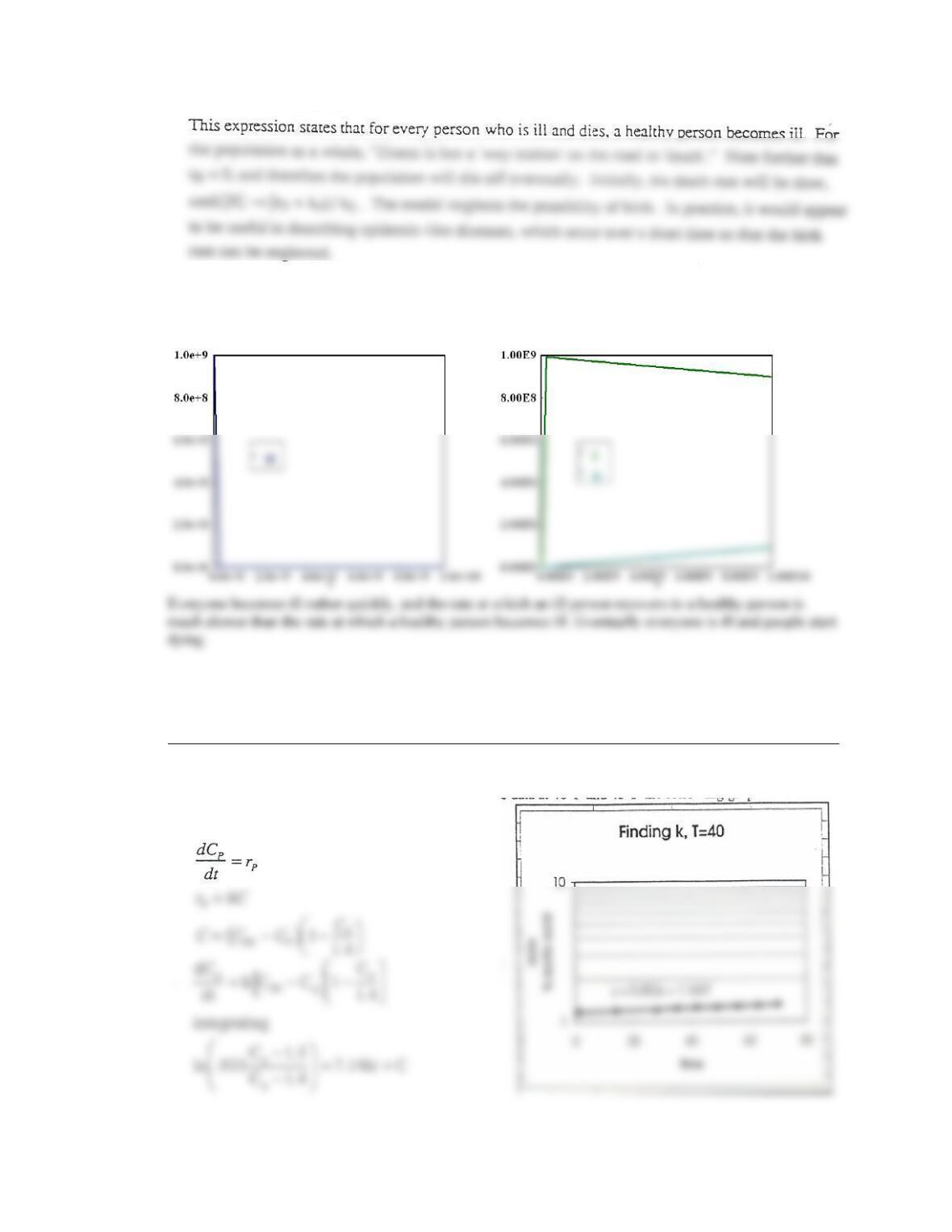
7-1
Solutions for Chapter 7 – Reaction Mechanisms,
Pathways, Bioreactions and Bioreactors
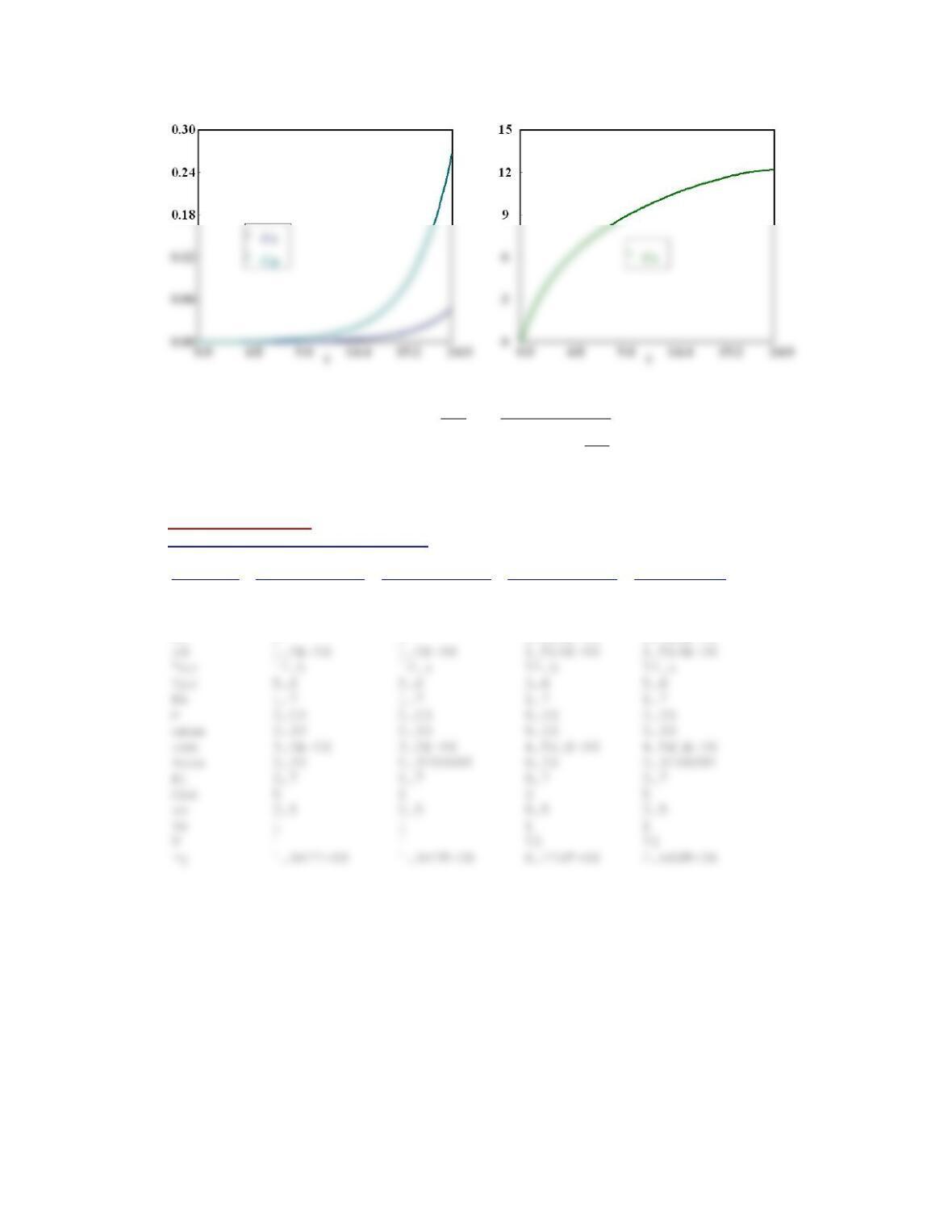
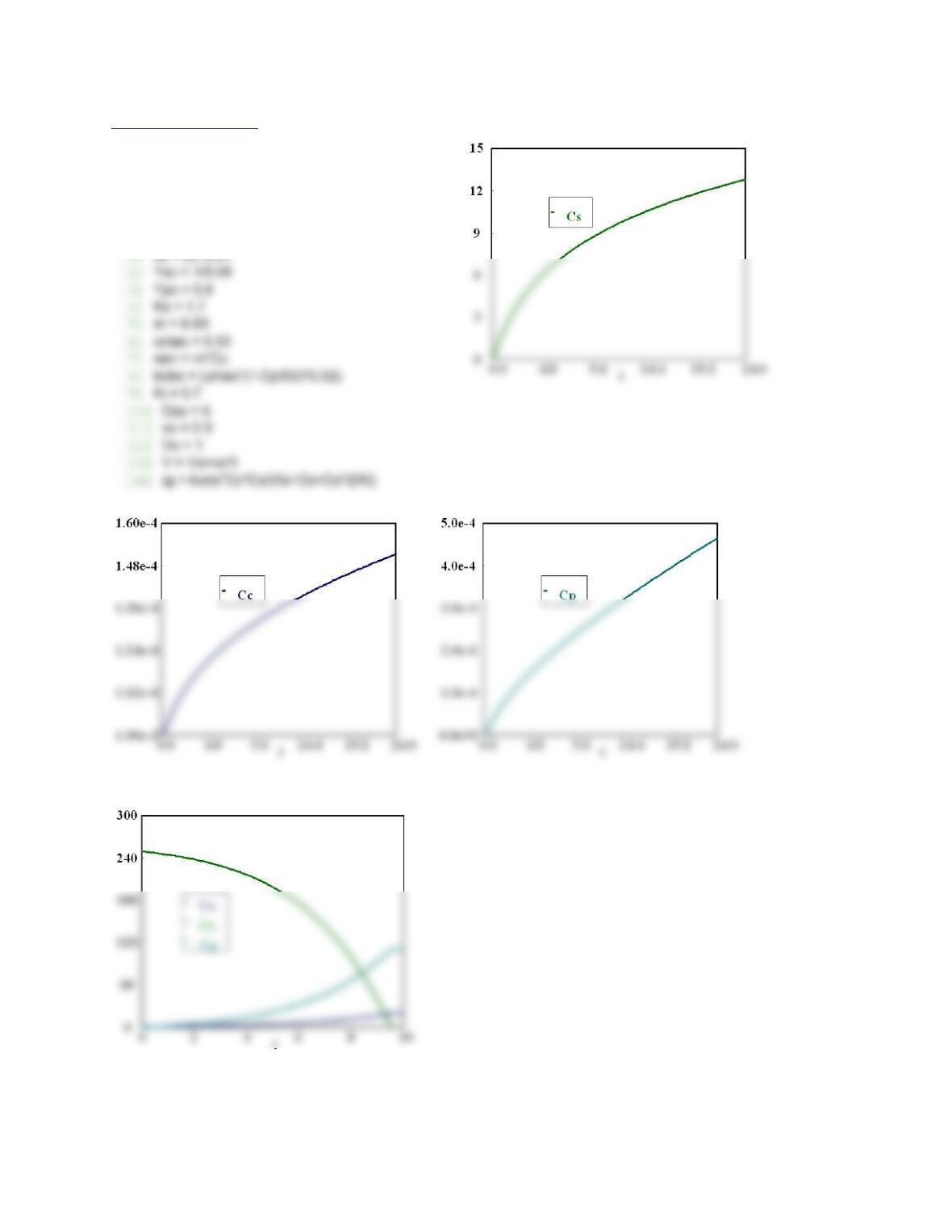
P7-1 (a) Example 7-1
The graph of Io/I will remain same if CS2 concentration changes. If concentration of M increases the slope
of line will decrease.
POLYMATH Results
Calculated values of the DEQ variables
Variable initial value minimal value maximal value final value
t 0 0 12 12
C1 0.1 2.109E-04 0.1 2.109E-04
C2 0 0 1.311E-09 1.311E-09
C6 0 0 3.602E-09 3.602E-09
ODE Report (STIFF)
Differential equations as entered by the user
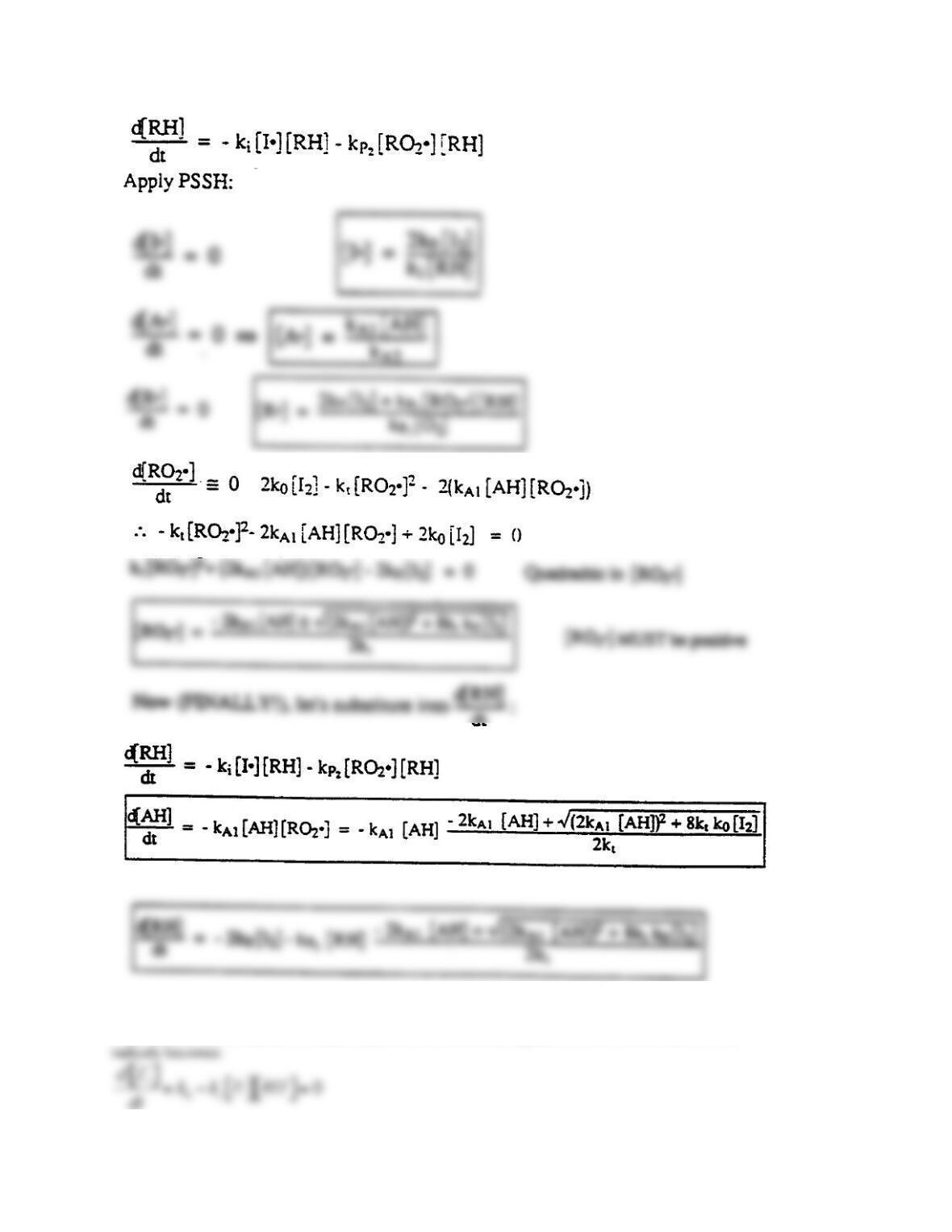
[1] d(C1)/d(t) = -k1*C1-k2*C1*C2-k4*C1*C6
[4] d(C4)/d(t) = k2*C1*C2-k3*C4+k4*C6*C1-
k5*C4^2
[5] d(C7)/d(t) = k4*C1*C6