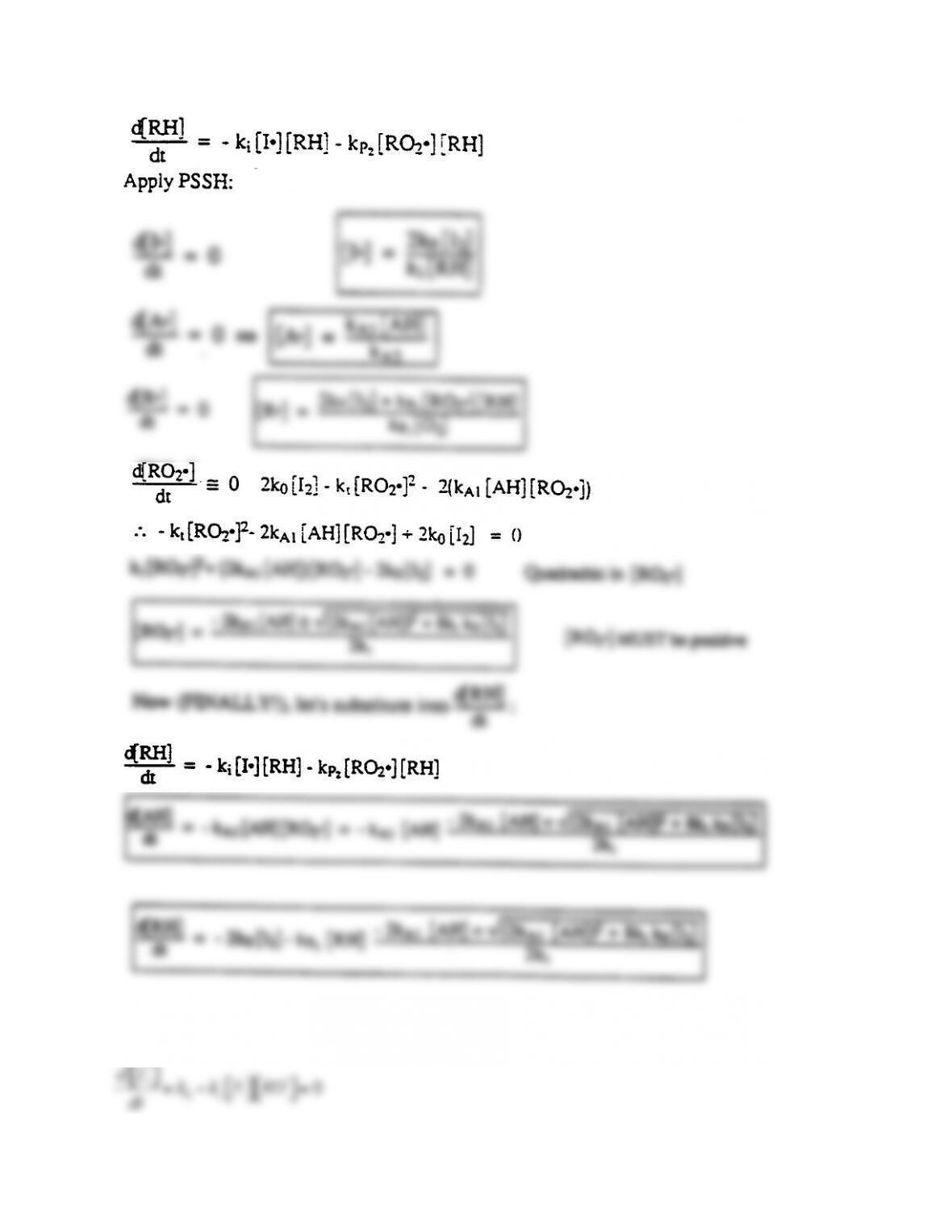
7-1
Solutions for Chapter 7 – Reaction Mechanisms,
Pathways, Bioreactions and Bioreactors
P7-1 (a) Example 7-1
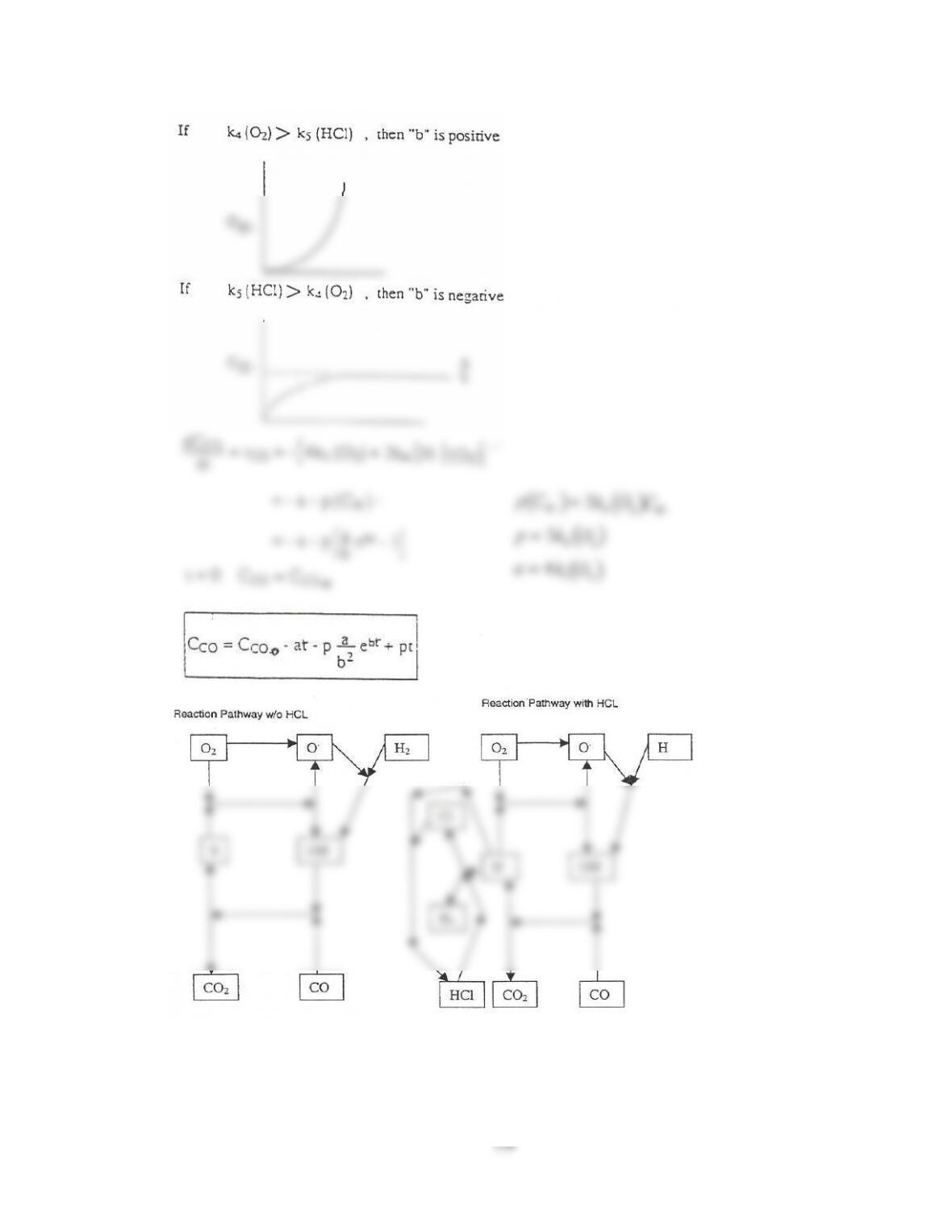
The graph of Io/I will remain same if CS2 concentration changes. If concentration of M increases the slope
P7-1 (b) Example 7-2
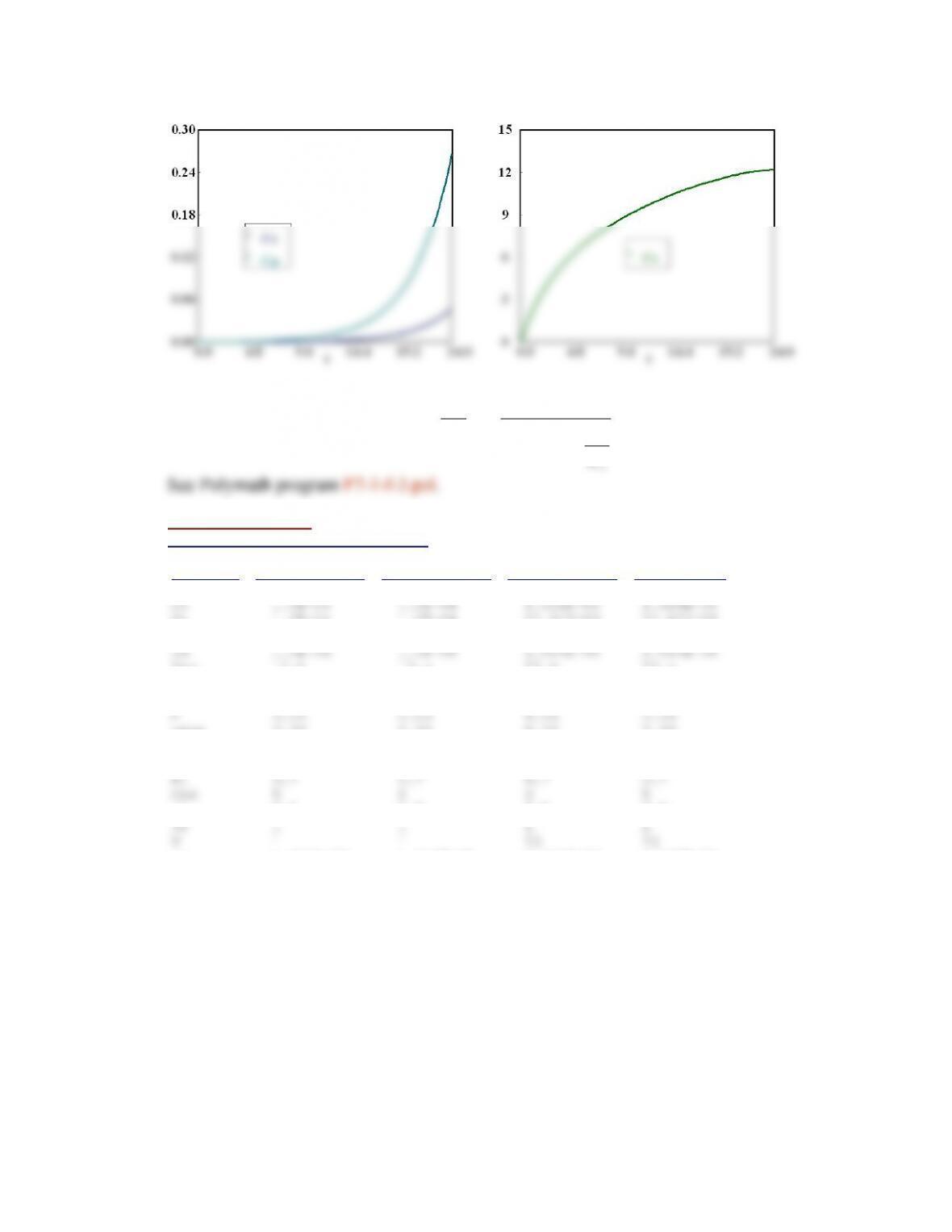
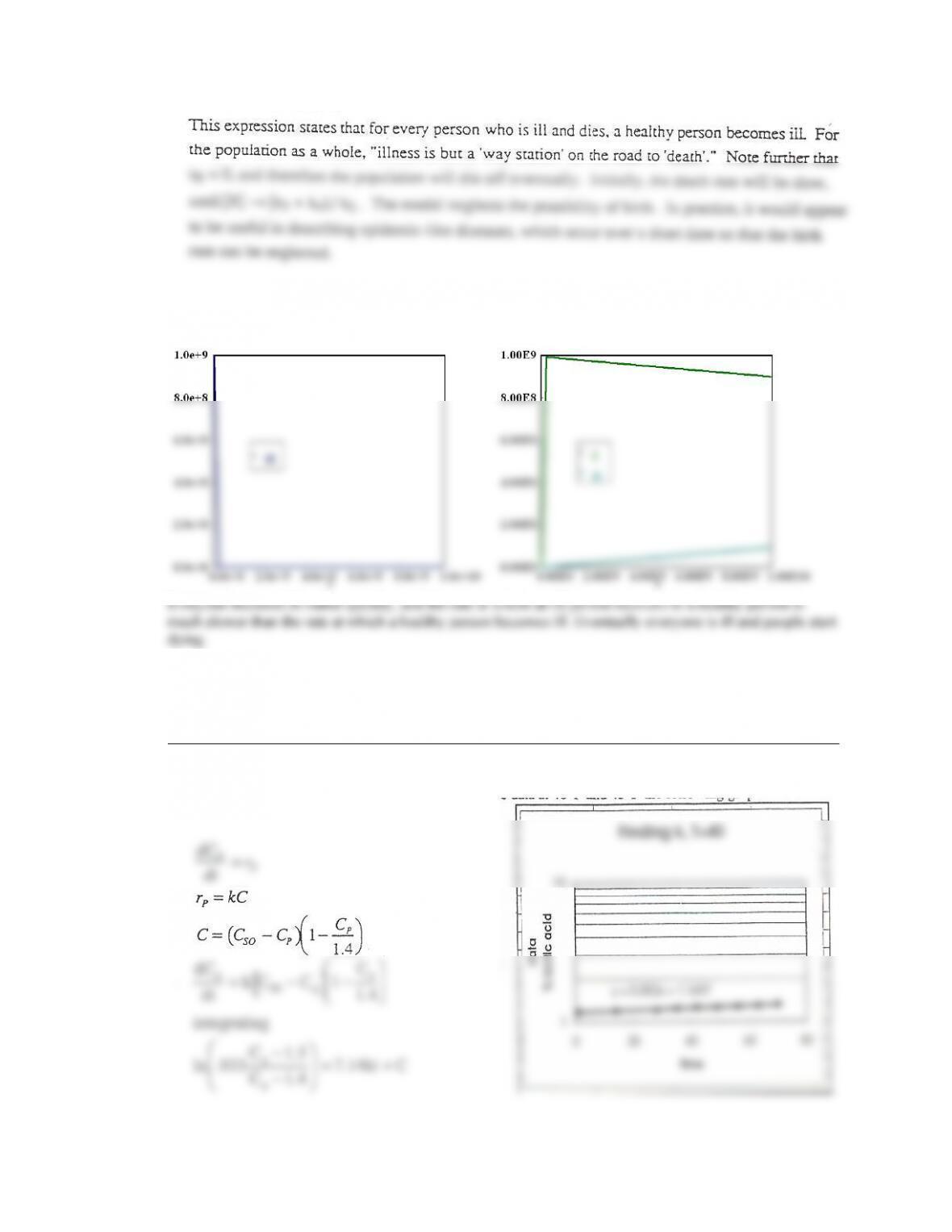
For t = 0 to t = 0.35 sec, PSSH is not valid as steady state not reached.
And at low temperature PSSH results show greatest disparity.
POLYMATH Results
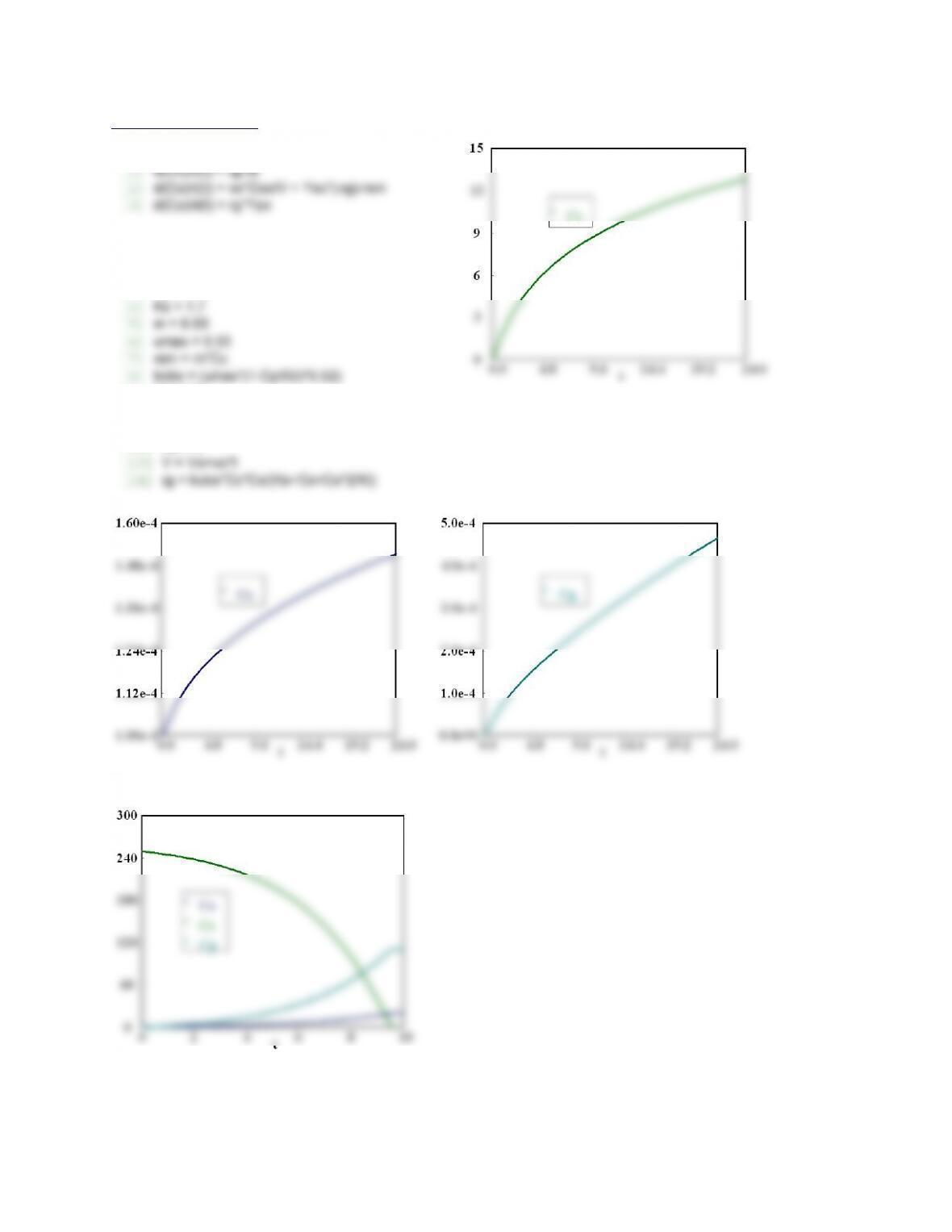
Calculated values of the DEQ variables
Variable initial value minimal value maximal value final value
t 0 0 12 12
C1 0.1 2.109E-04 0.1 2.109E-04
C2 0 0 1.311E-09 1.311E-09
C6 0 0 3.602E-09 3.602E-09
C4 0 0 2.665E-07 1.276E-08
k5 3.98E+09 3.98E+09 3.98E+09 3.98E+09
k2 2.283E+06 2.283E+06 2.283E+06 2.283E+06
k4 9.53E+08 9.53E+08 9.53E+08 9.53E+08
k3 5.71E+04 5.71E+04 5.71E+04 5.71E+04
ODE Report (STIFF)
Differential equations as entered by the user
[1] d(C1)/d(t) = -k1*C1-k2*C1*C2-k4*C1*C6
[6] d(C3)/d(t) = k2*C1*C2
[7] d(C5)/d(t) = k3*C4