6-9
P6-2 (k)
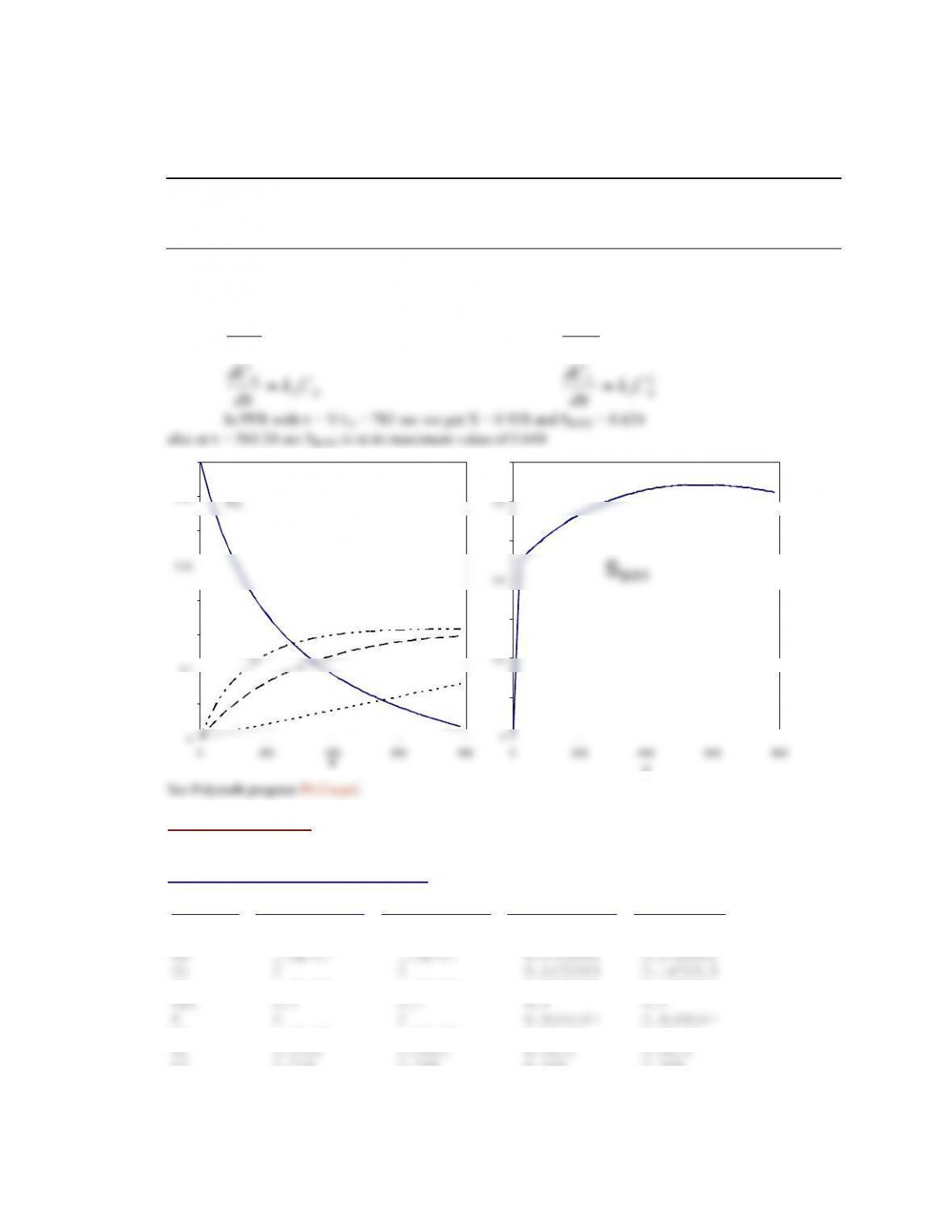
Base case Equal-molar feed
un-reacted mesitylene.
P6-2 (l) Individualized solution
P6-3 Solution is in the decoding algorithm given with the modules ( ICM problem )
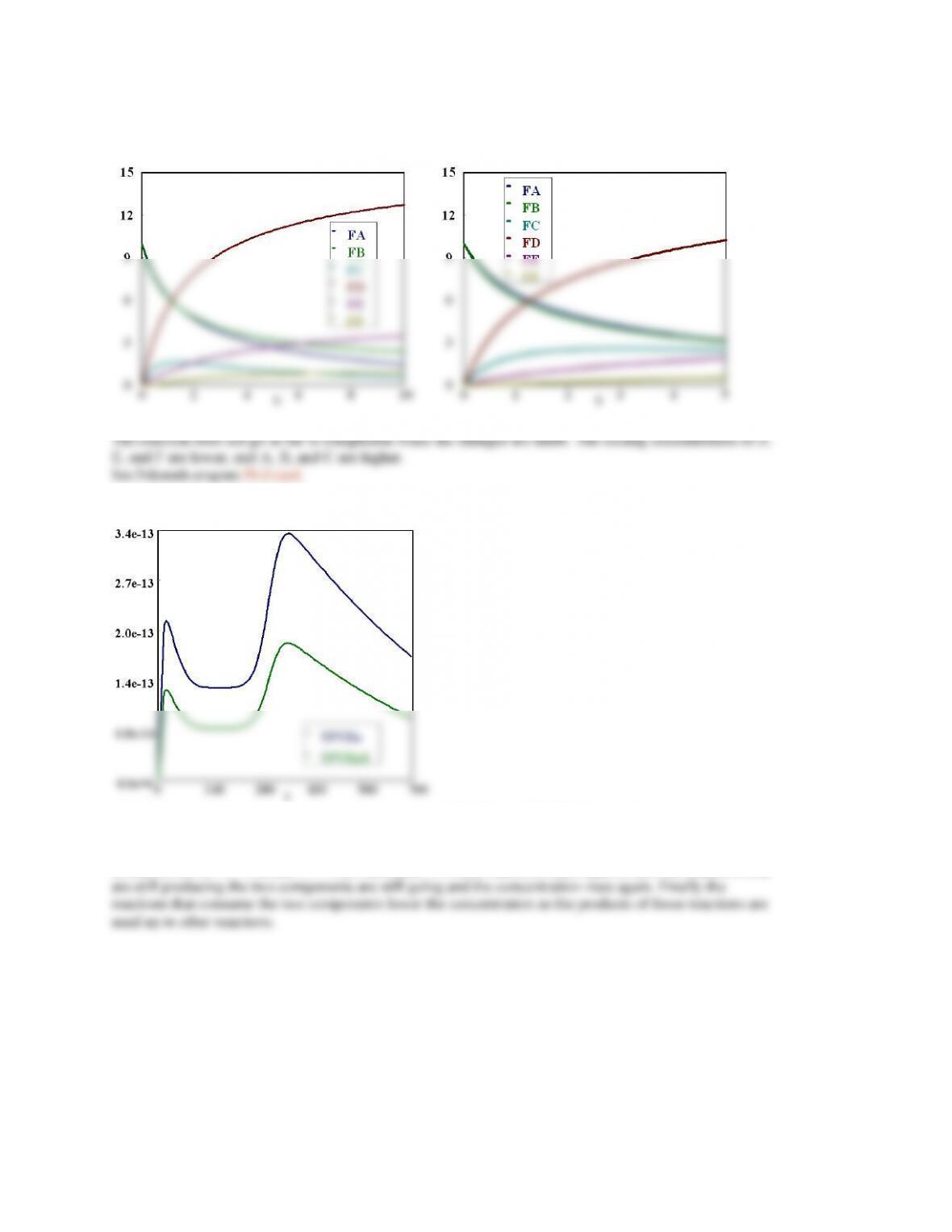
P6-4 (a)
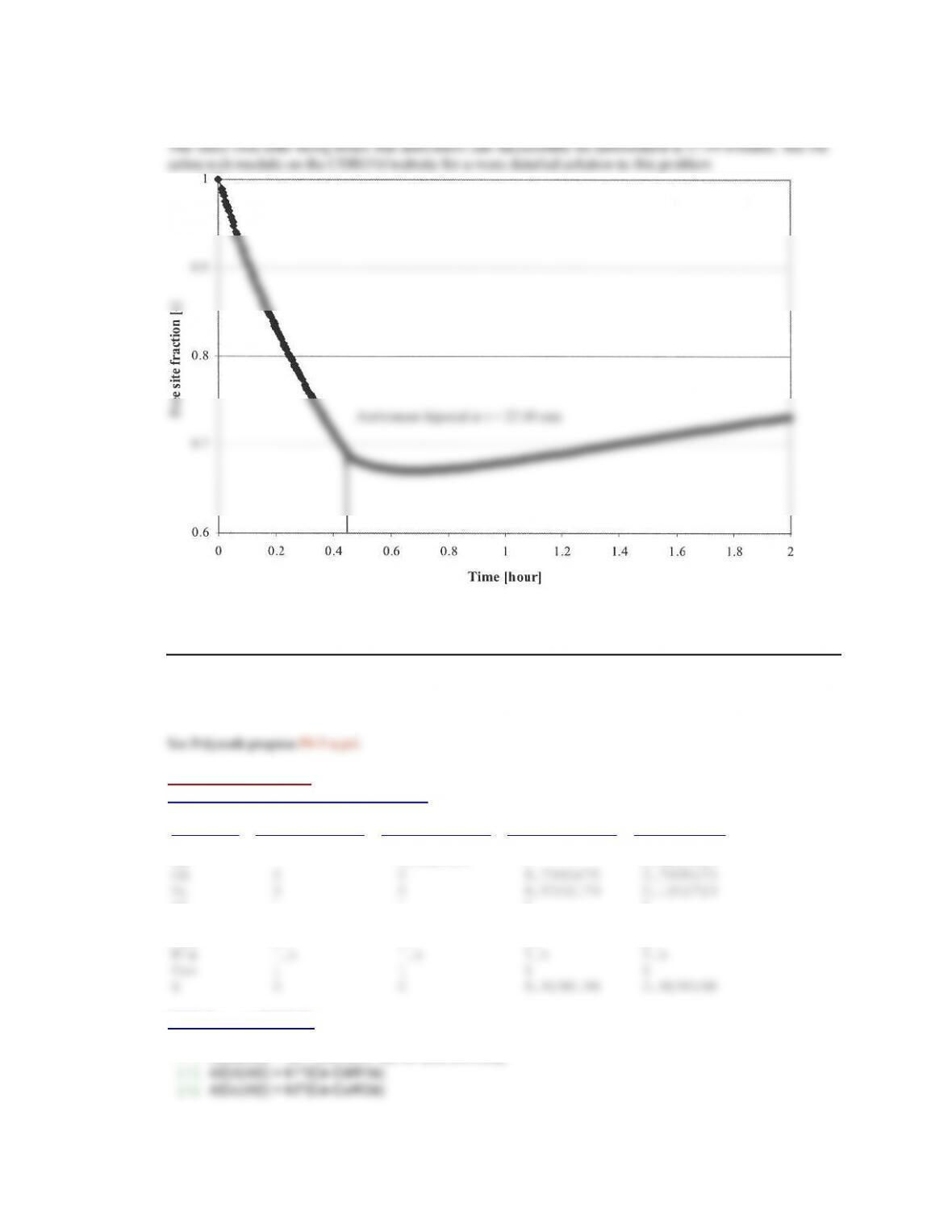
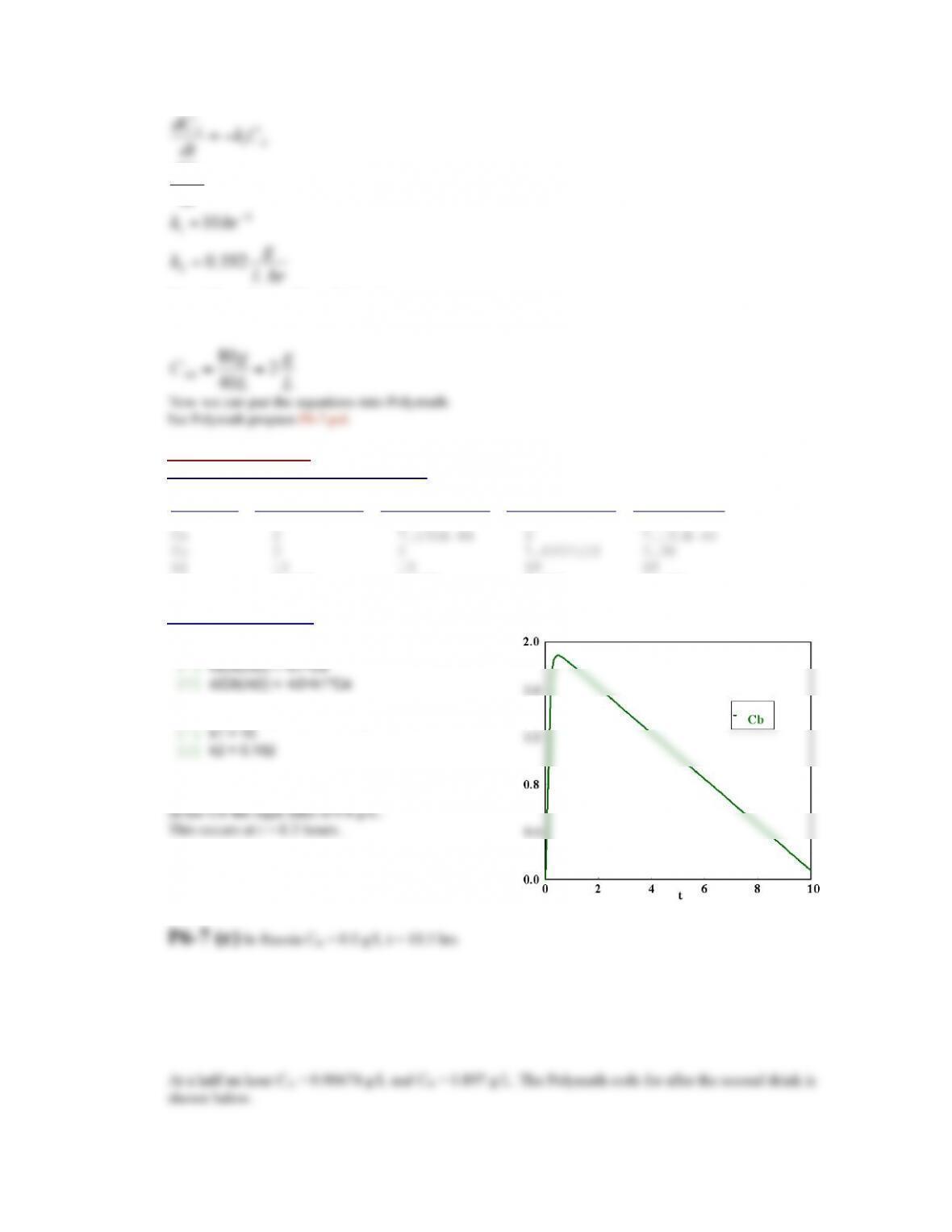
Assume that all the bites will deliver the standard volume of venom. This means that the initial
concentration increases by 5e-9 M for every bite.
After 11 bites, no amount of antivenom can keep the number of free sites above 66.7% of total sites. This
means that the initial concentration of venom would be 5.5e-8 M. The best result occurs when a dose of
antivenom such that the initial concentration of antivenom in the body is 5.7e-8 M, will result in a
P6-4 (b)
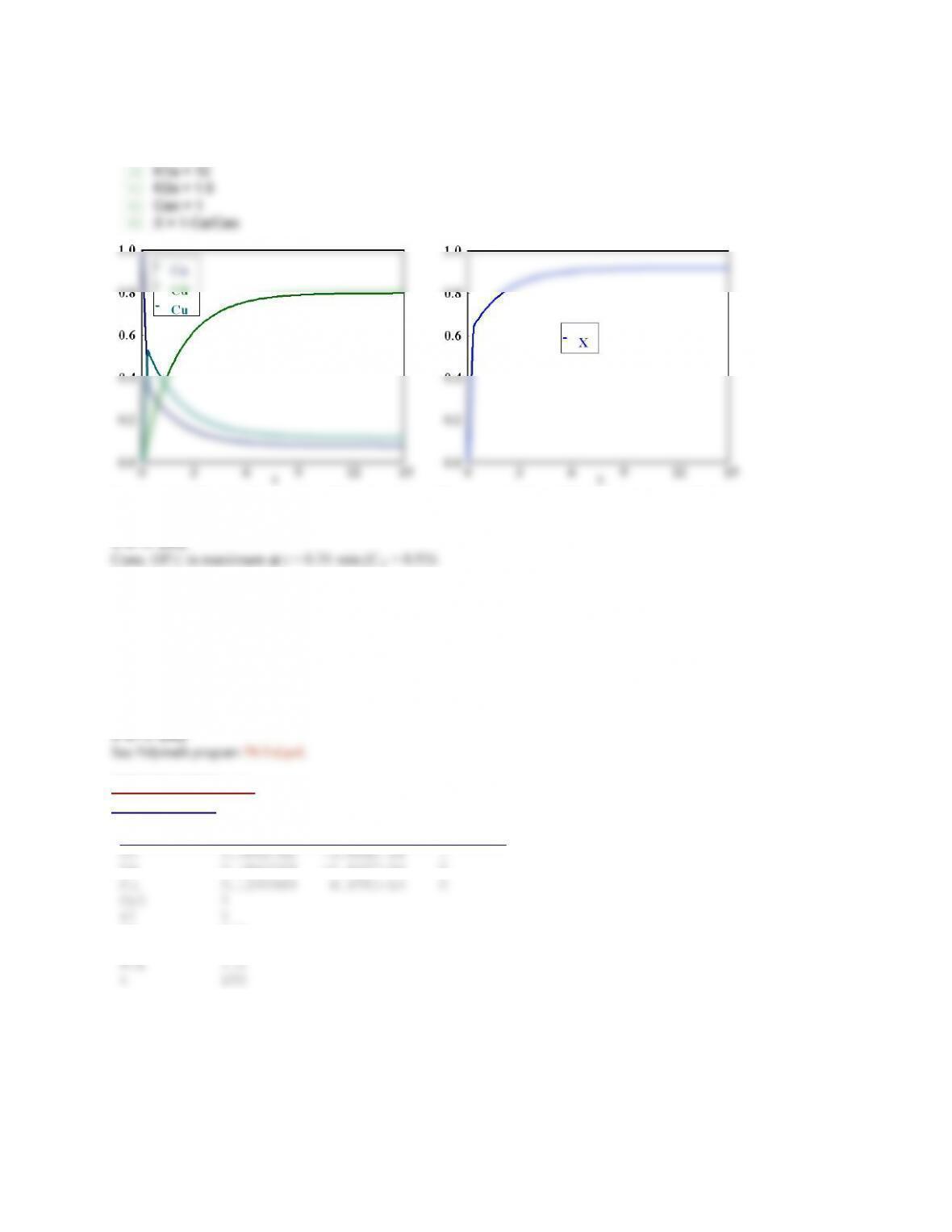
The victim was bitten by a harmless snake and antivenom was injected. This means that the initial
concentration of venom is 0. From the program below, we see that if an amount of antivenom such that the