4-63
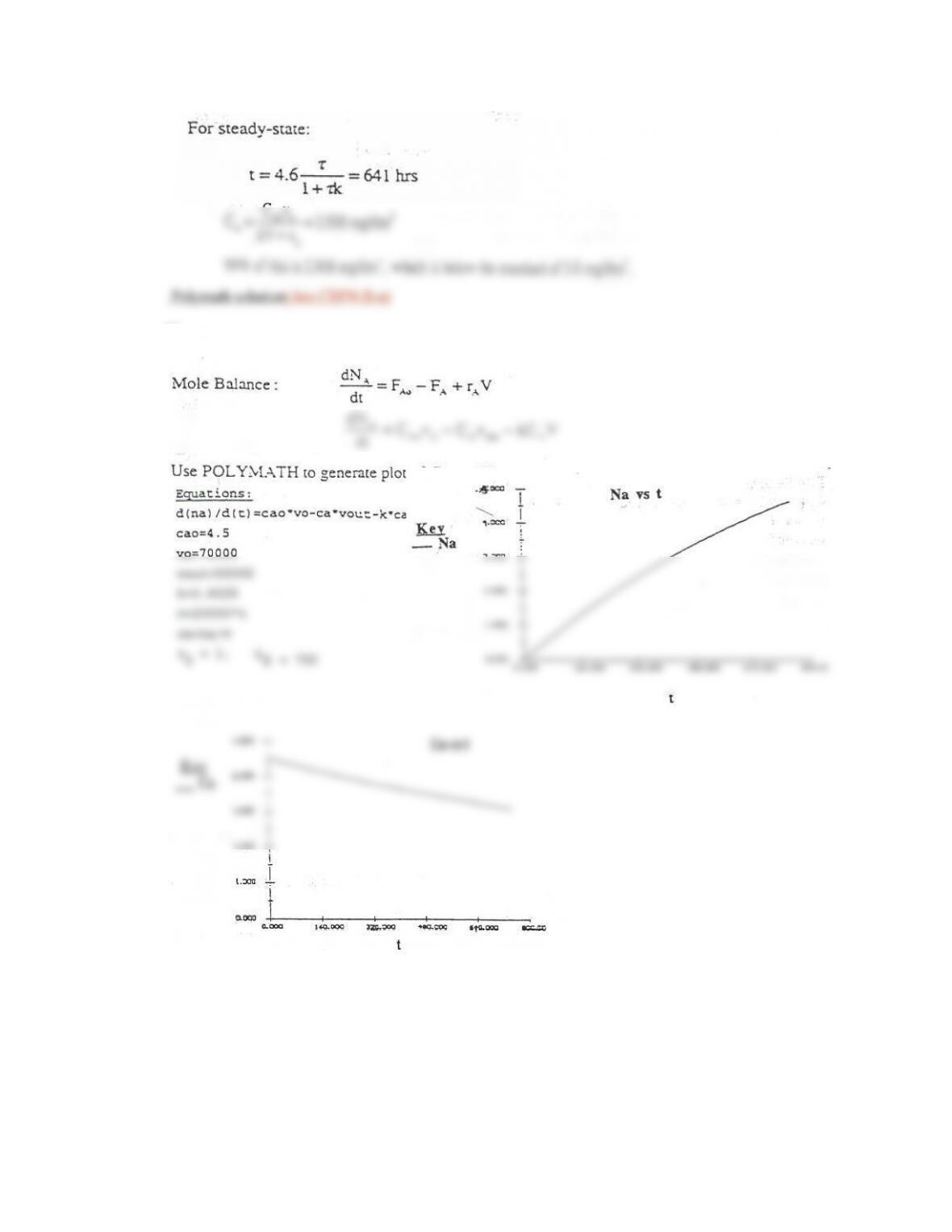
POLYMATH Results
Calculated values of the DEQ variables
Variable initial value minimal value maximal value final value
t 0 0 100 100
Ca1 0 0 1.1484734 1.1484734
Ca2 0 0 0.7281523 0.7281523
Ca3 0 0 0.6278144 0.6278144
k 0.025 0.025 0.025 0.025
Cao 2 2 2 2
ODE Report (RKF45)
Differential equations as entered by the user
[1] d(Ca1)/d(t) = (2*Cao/3 -Ca1)/tau -k*Ca1*Cb1
[2] d(Ca2)/d(t) = Ca1/tau - Ca2/tau2 -k*Ca2*Cb2
[3] d(Ca3)/d(t) = (Ca2 - Ca3)/tau2 -k*Ca3*Cb3
Explicit equations as entered by the user
[1] k = 0.025
[2] Cao = 2
[3] tau = 200/15
[4] X = 1 - 2*Ca3/Cao
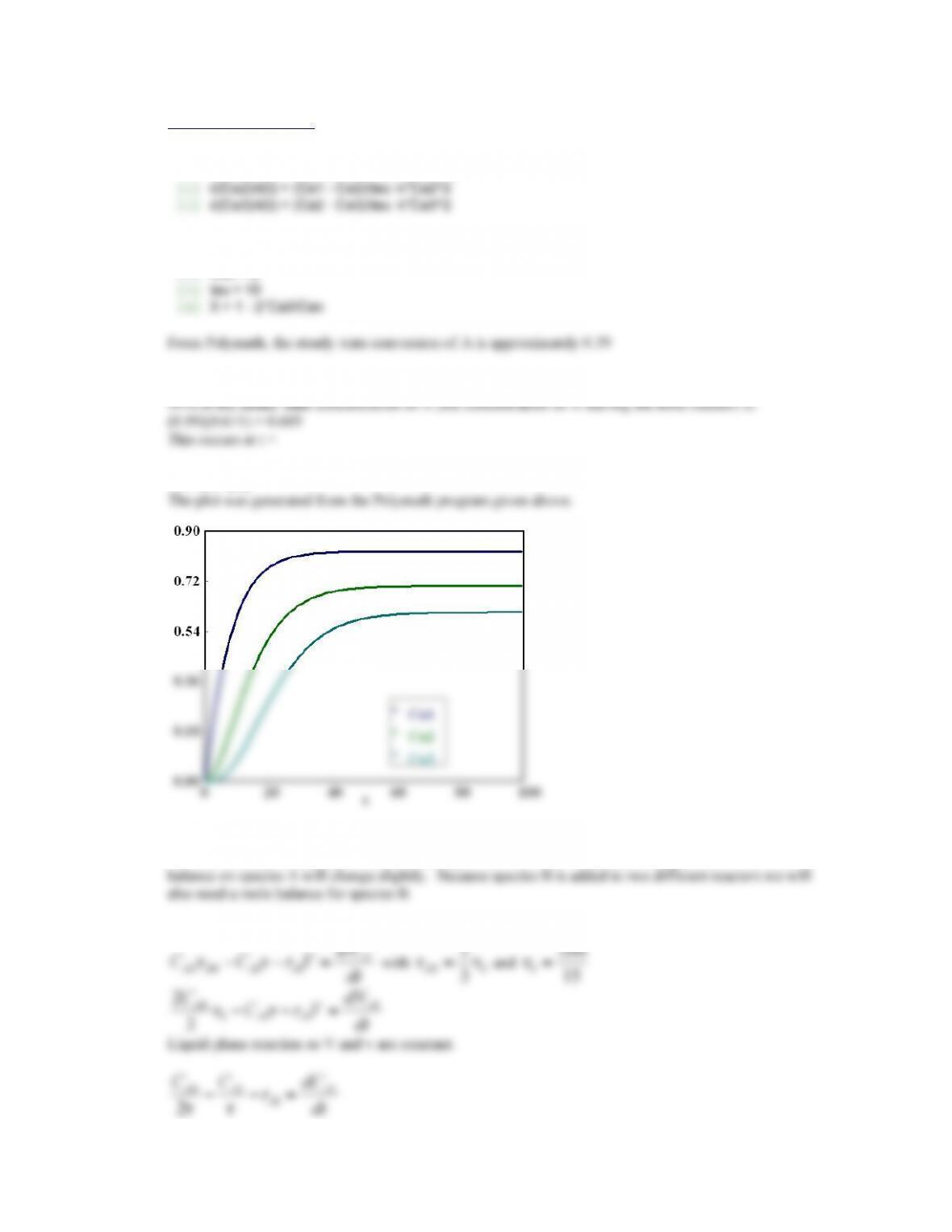
Equilibrium conversion is 0.372.
This conversion is reached at t = 85.3 minutes.
P4-33 (e) Individualized solution