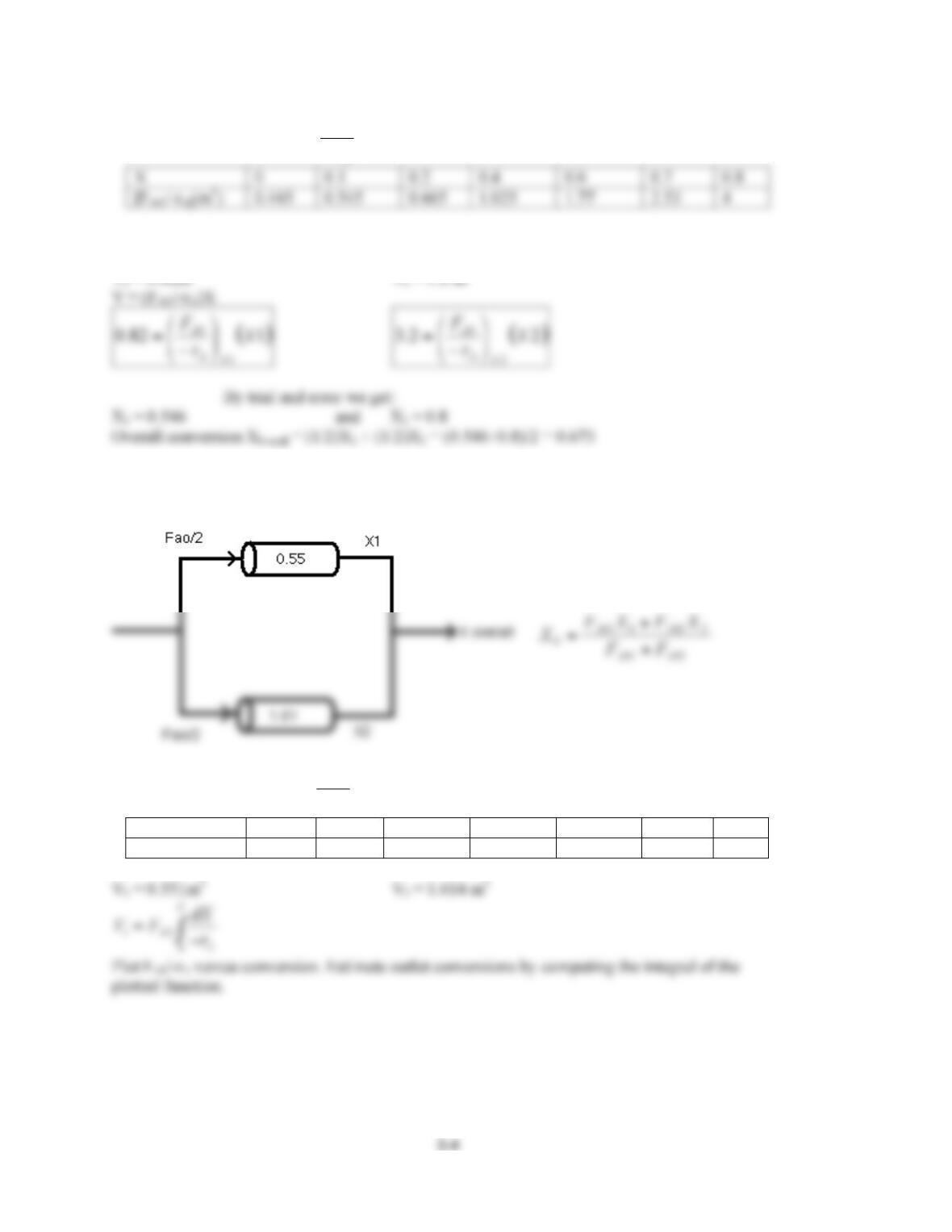
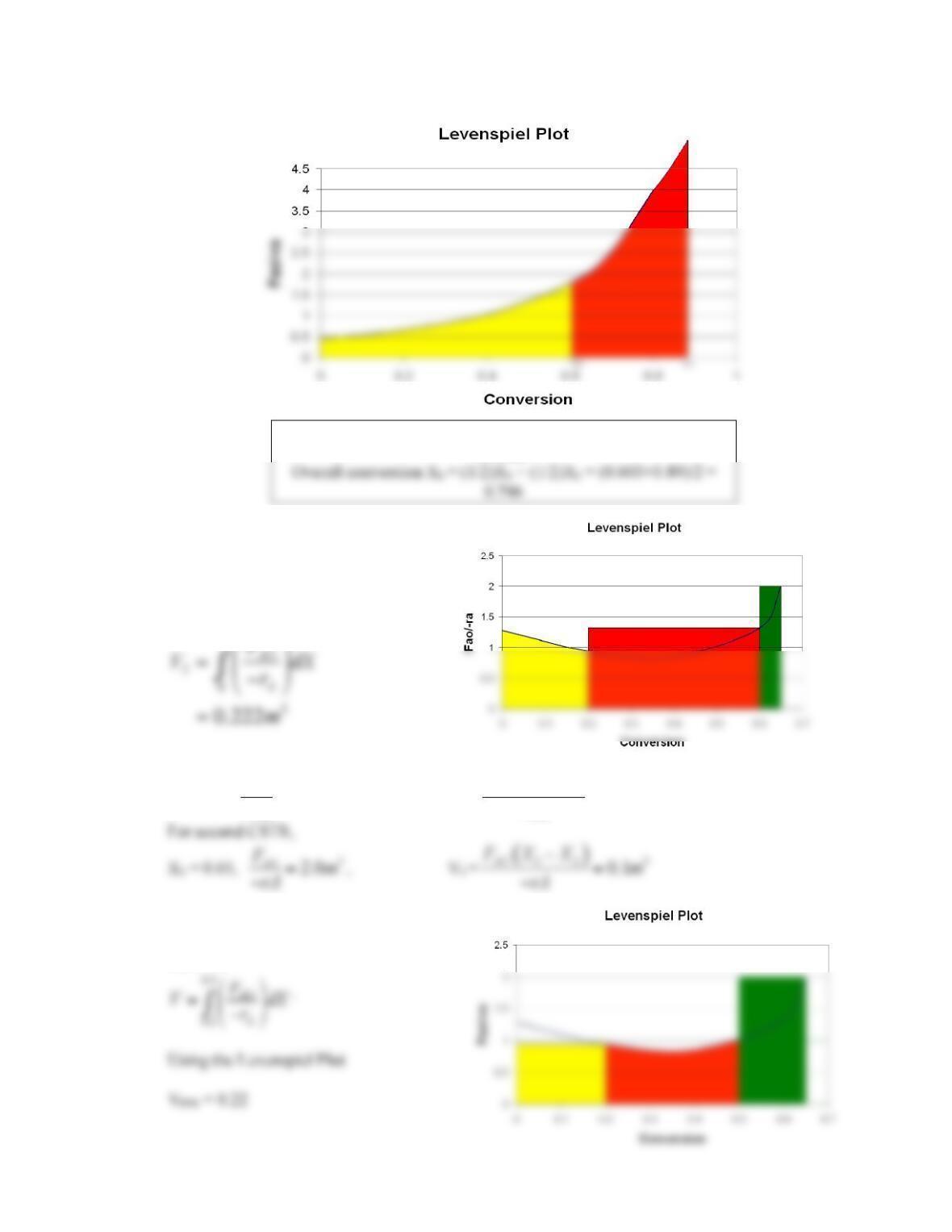
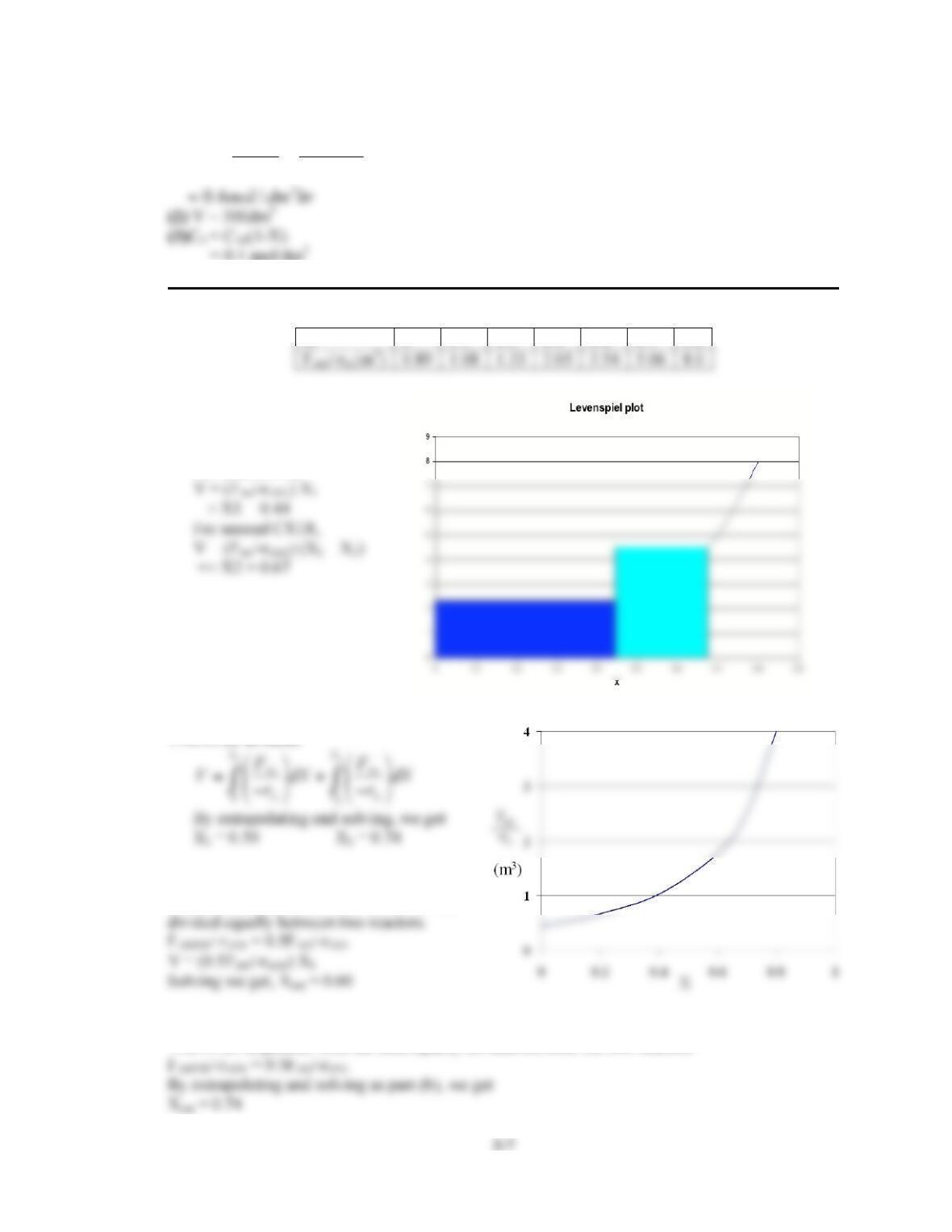
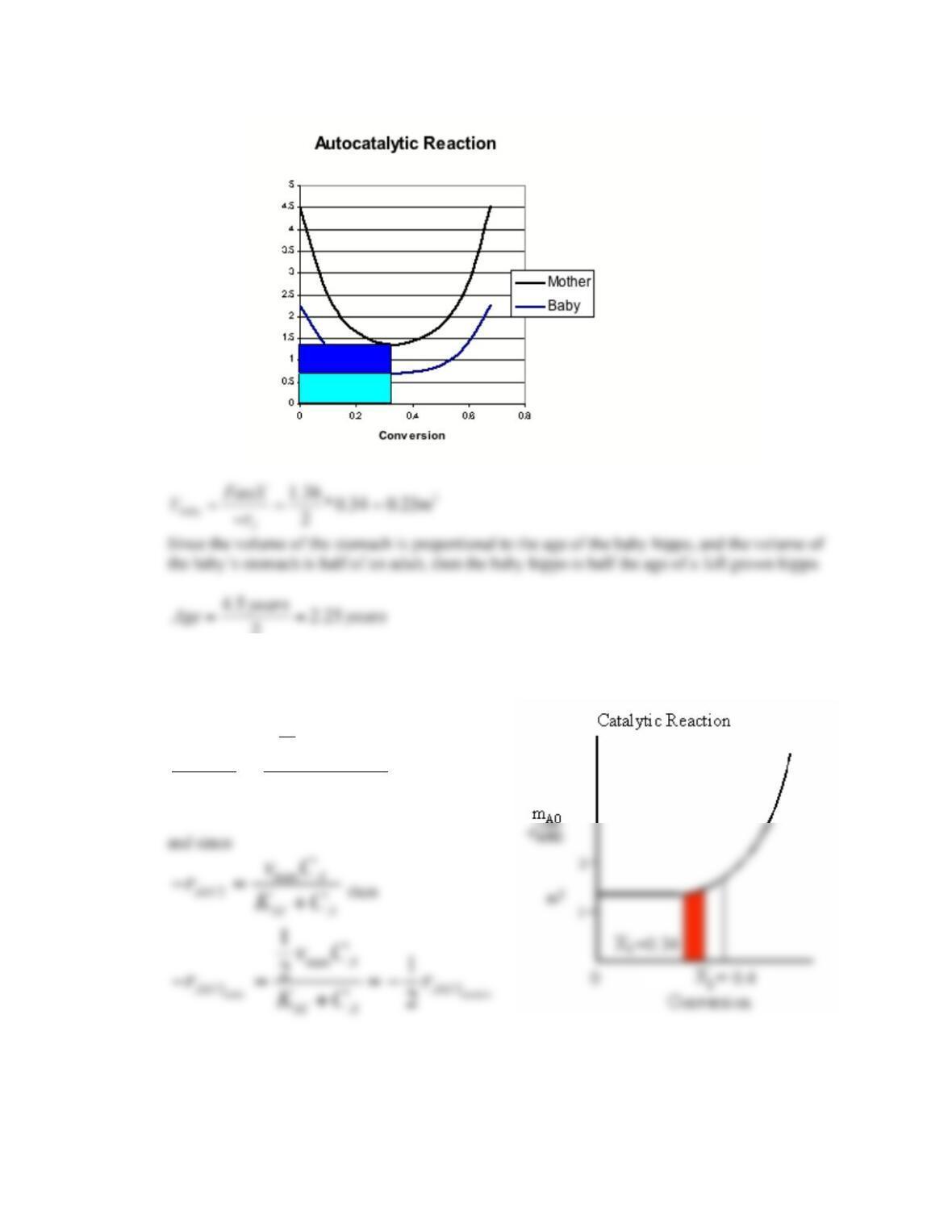
2-1
Solutions for Chapter 2 - Conversion and Reactor
Sizing
Synopsis
General: The overall goal of these problems is to help the student realize that if they
have –rA=f(X) they can “design” or size a large number of reaction systems. It sets the
stage for the algorithm developed in Chapter 4.
P2-1. This problem will keep students thinking about writing down what they learned
every chapter.
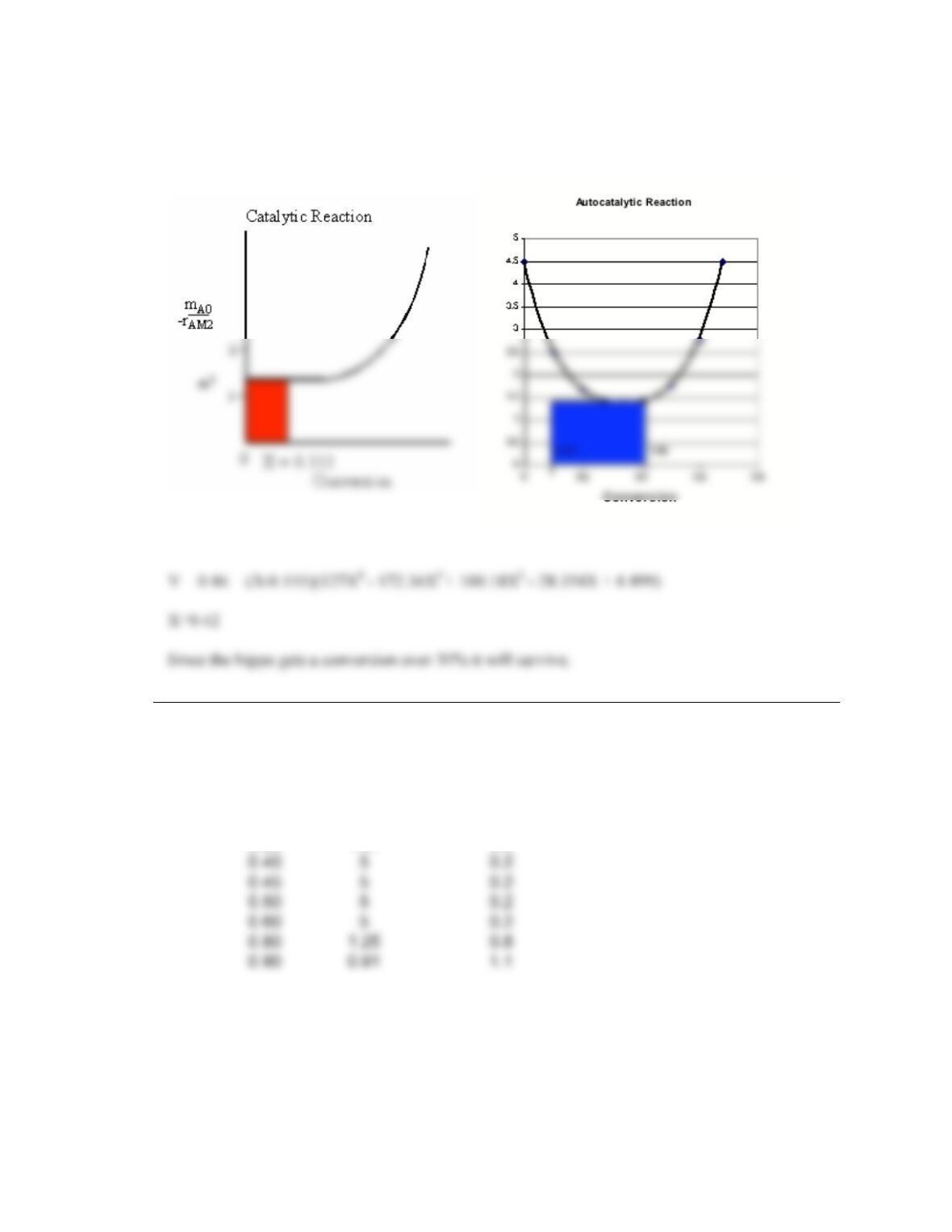
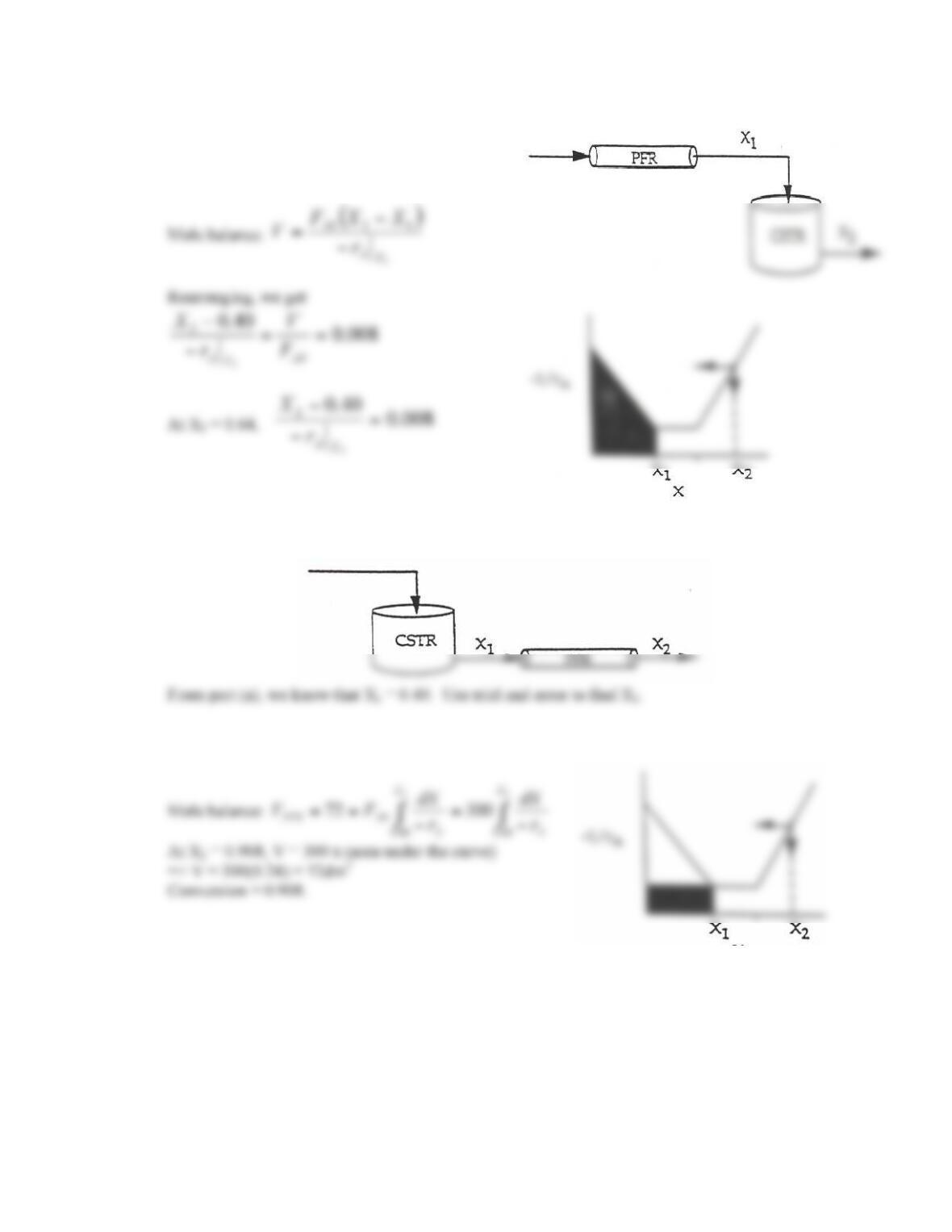
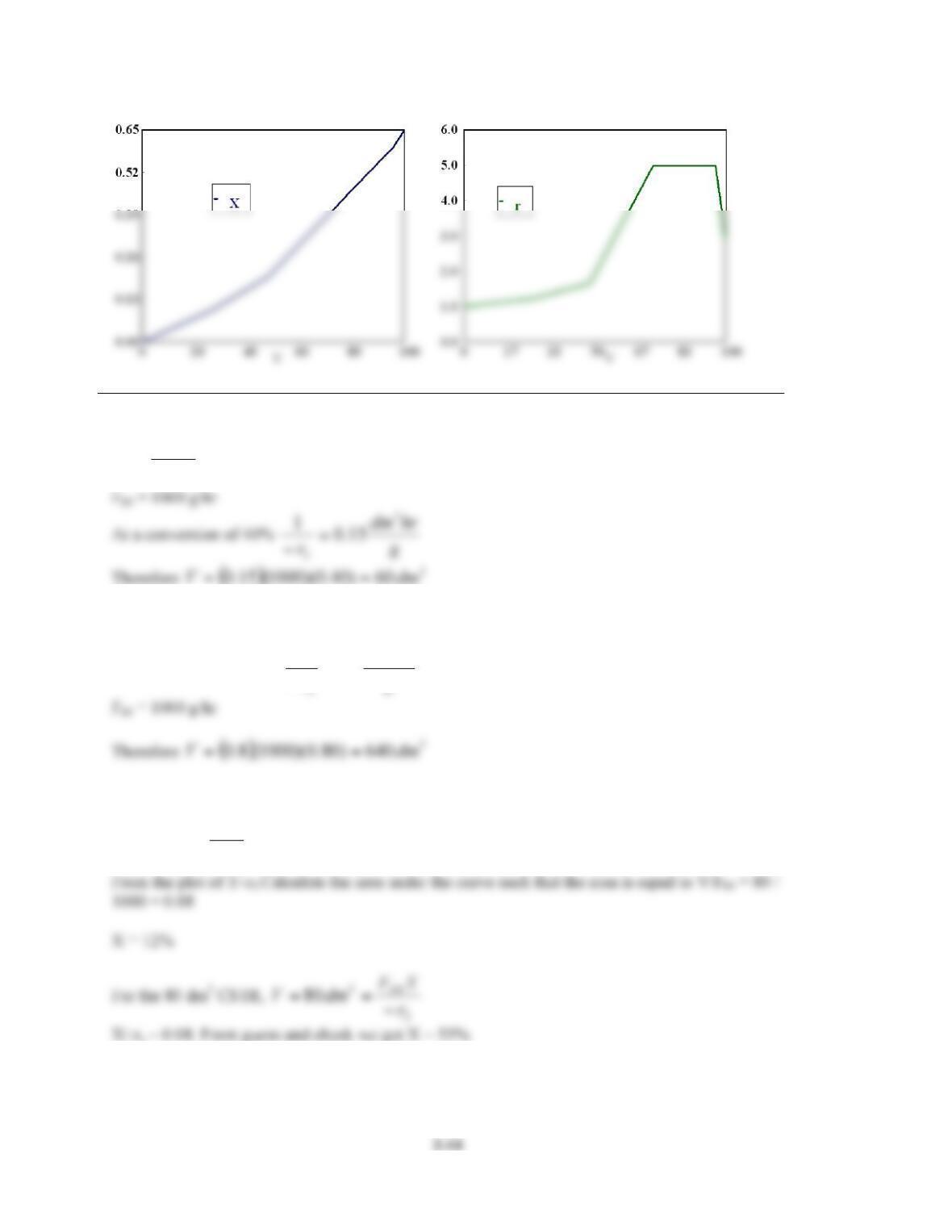
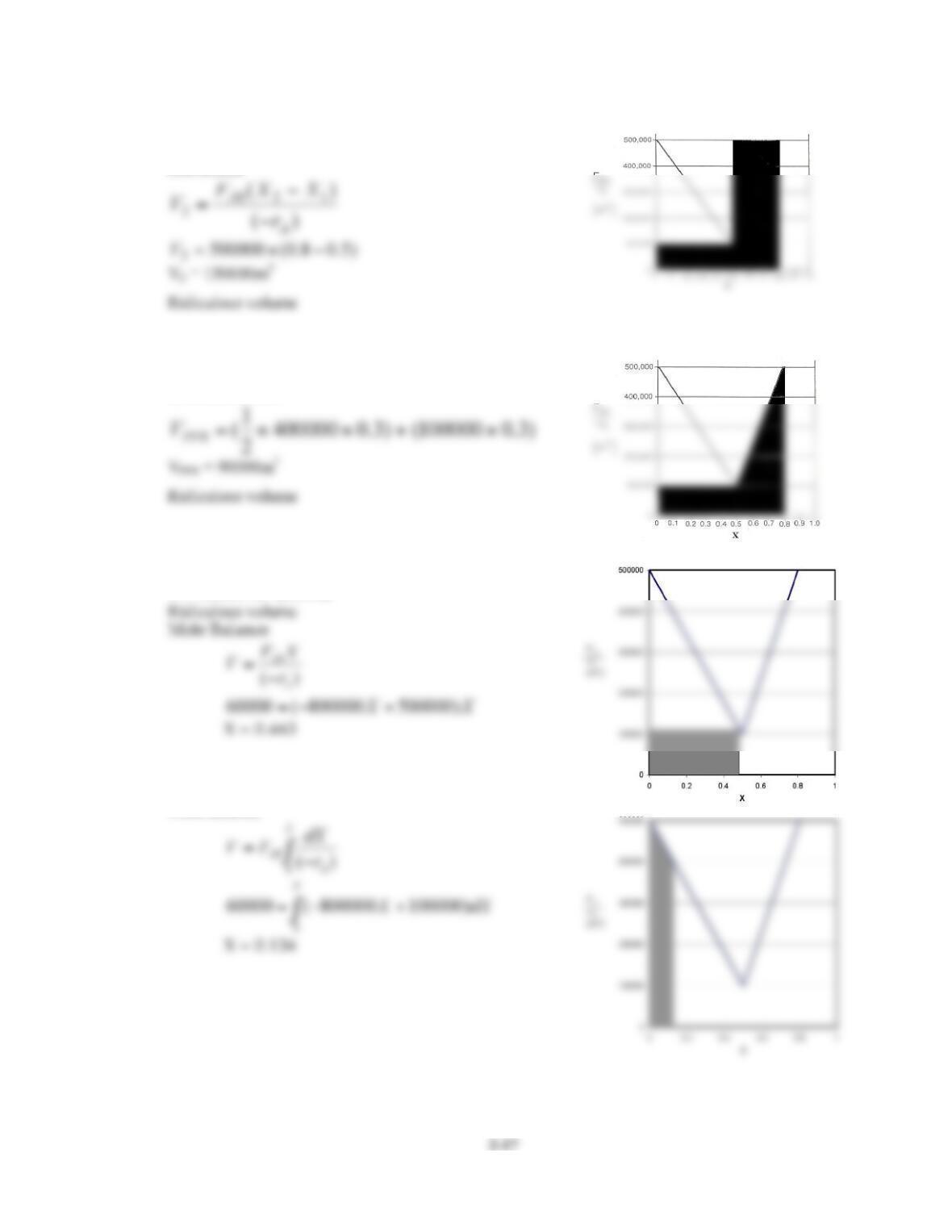
P2-5. This is a reasonably challenging problem that reinforces Levenspiels plots.
P2-6. Novel application of Levenspiel plots from an article by Professor Alice Gast at
Massachusetts Institute of Technology in CEE.
P2-7. Straight forward problem alternative to problems 8, 9, and 12.
P2-11. Great motivating problem. Students remember this problem long after the
course is over.