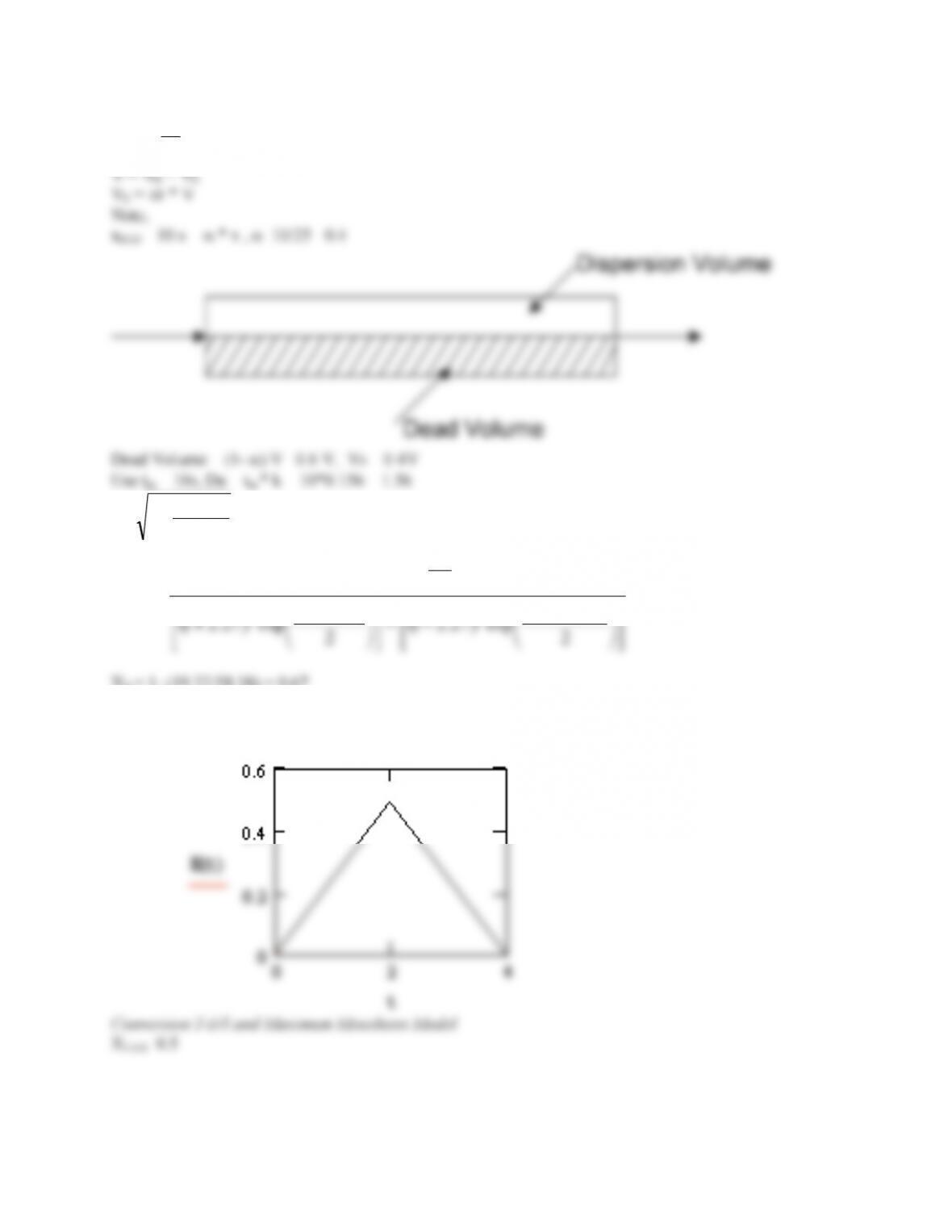
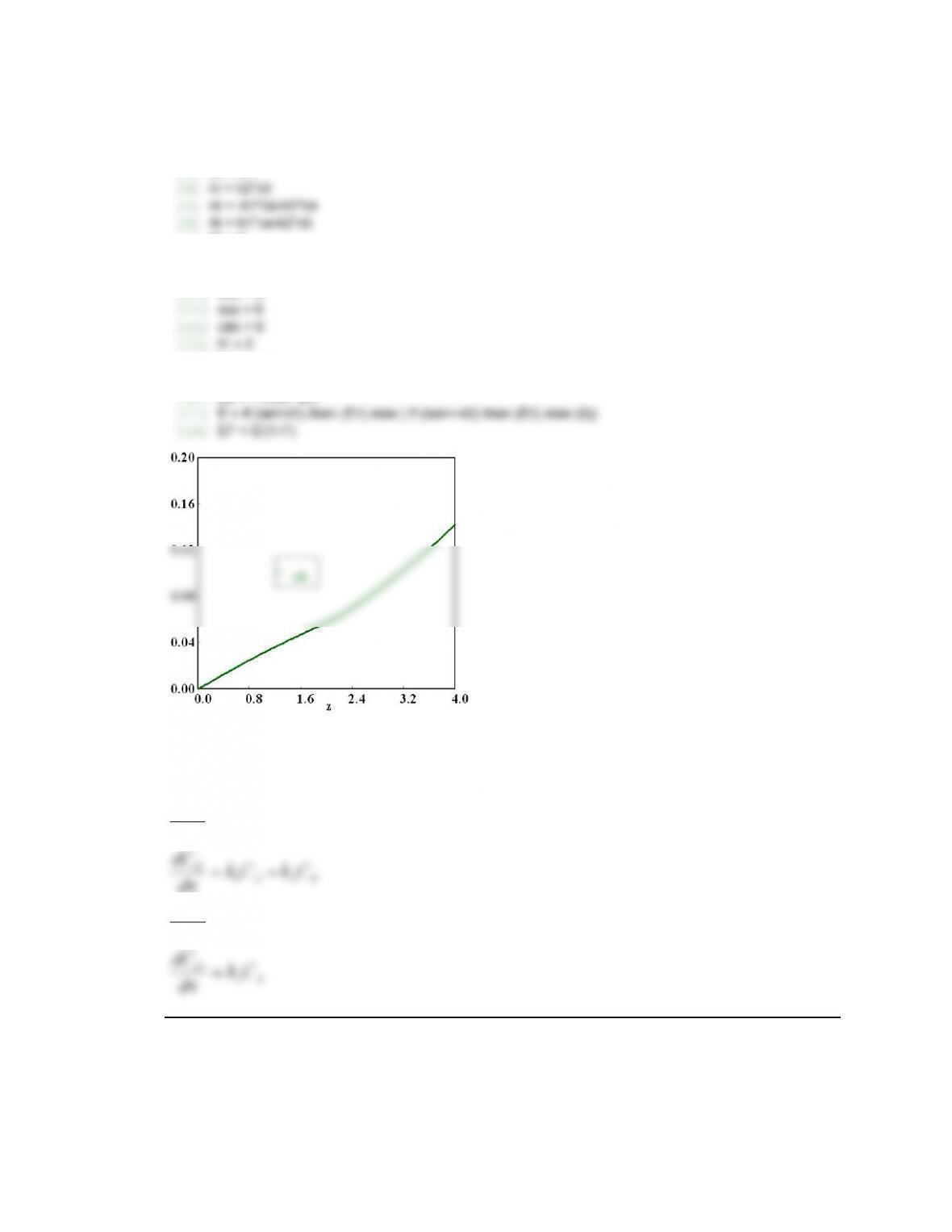
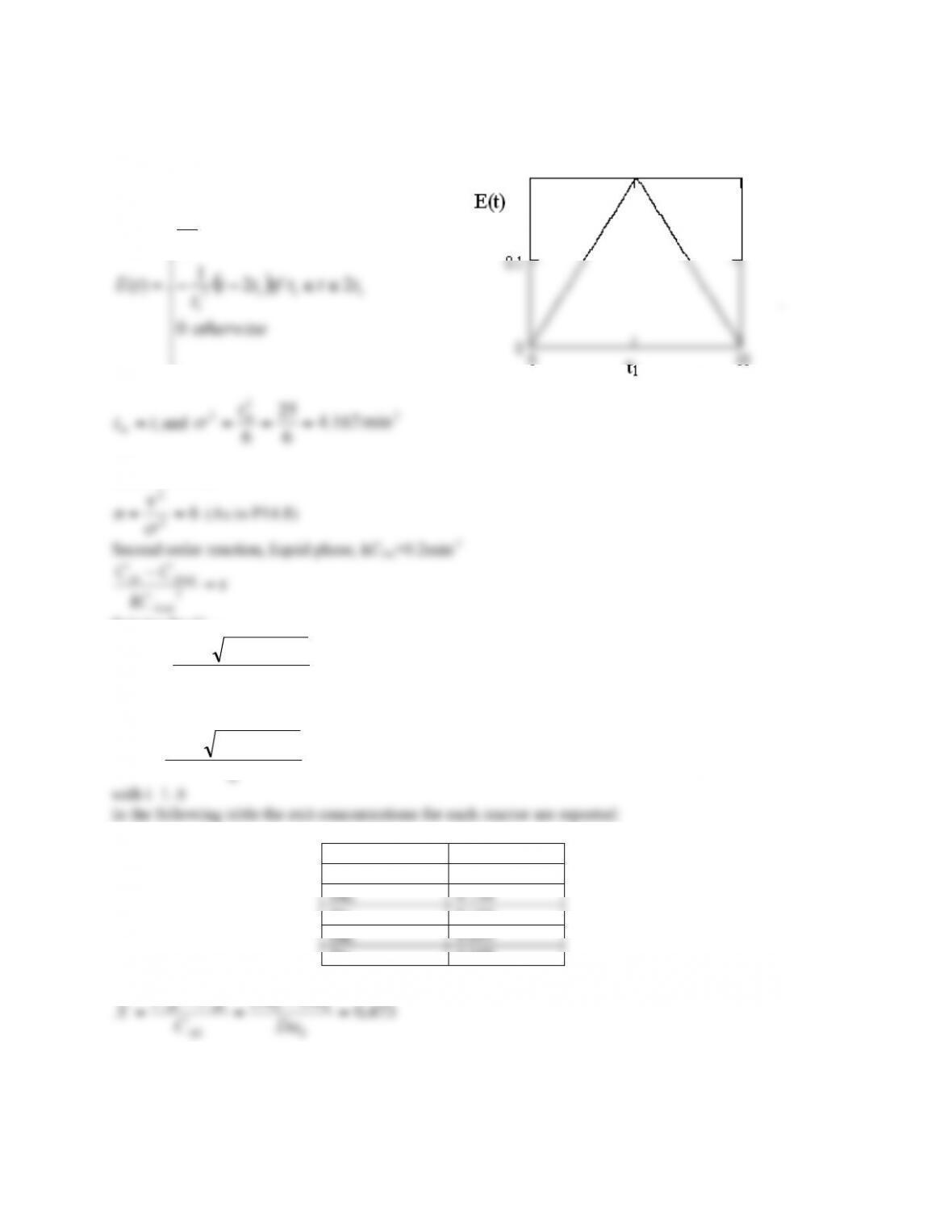
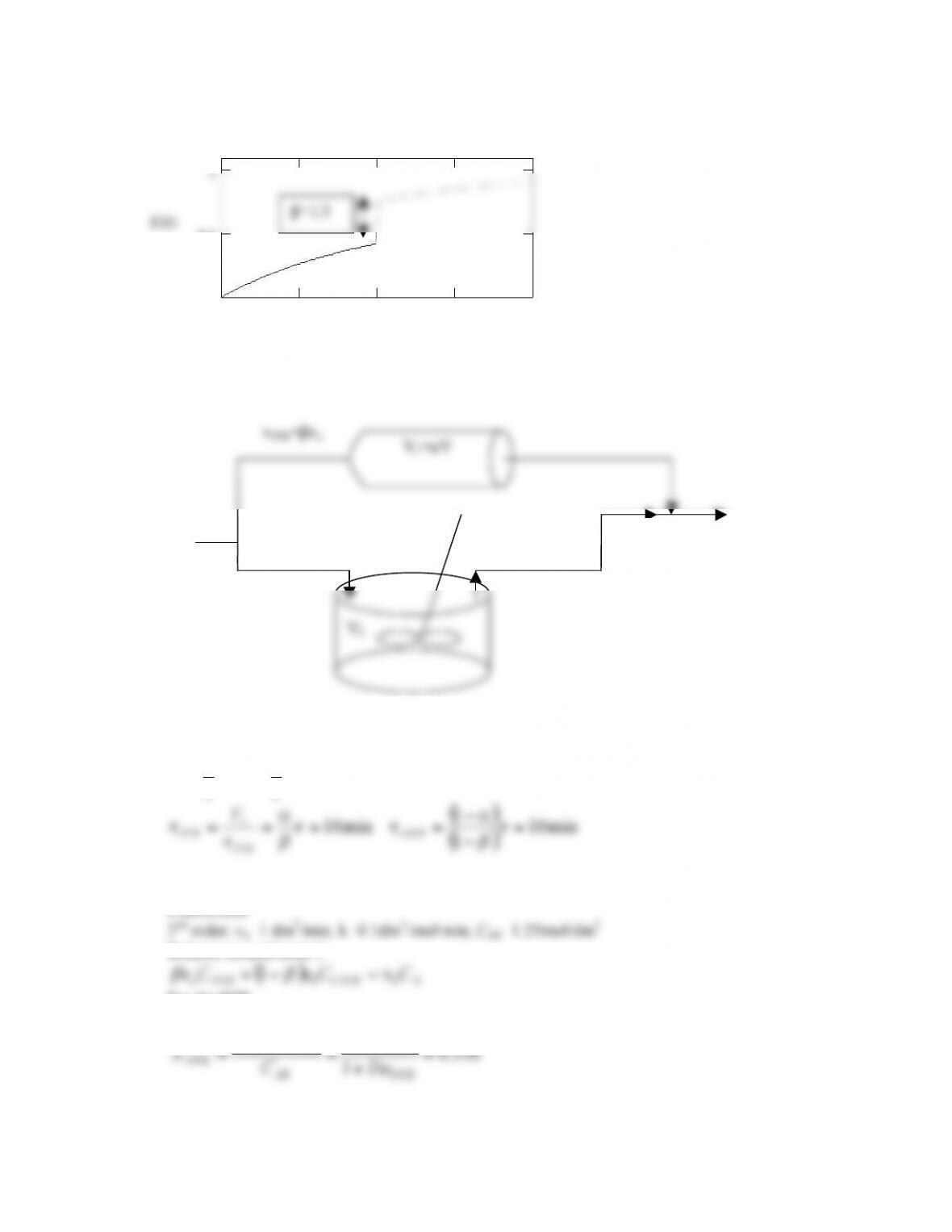
POLYMATH Results
Calculated values of the DEQ variables
Variable initial value minimal value maximal value final value
z 0 0 60 60
X 0 0 0.4773052 0.4047103
F 0.99 -0.010344 0.99 -0.010344
Cao 1 1 1 1
lam 60 0 60 0
Ca 1 0.52273 1 0.5952897
k 0.1 0.1 0.1 0.1
E2 -9.8680524 -9.8680524 0.092343 0.092343
E3 2.228E-05 1.806E-05 0.083717 0.083717
ODE Report (RKF45)
Differential equations as entered by the user
[1] d(X)/d(z) = -(ra/Cao+E/(1-F)*X)
[2] d(F)/d(z) = -E
Explicit equations as entered by the user
[1] Cao = 1
[2] lam = 60-z
[3] Ca = Cao*(1-X)
[4] k = .1
[5] ra = -k*Ca^2
[7] E1 = -0.0011675*lam^4+0.011355*lam^3-0.047492*lam^2+0.0995005*lam
[8] E2 = -1.8950*10^(-6)*lam^4+8.7202*10^(-5)*lam^3-1.1739*10^(-3)*lam^2-1.7979*10^(-
4)*lam+0.092343
[9] E3 = 1.2618*10^(-8)*lam^4-2.4995*10^(-6)*lam^3+1.8715*10^(-4)*lam^2-6.3512*10^(-
[10] E = if(lam<=3)then(E1)else(if(lam<=20)then(E2)else(if(lam<60)then(E3)else(E4)))
(Check F(t))
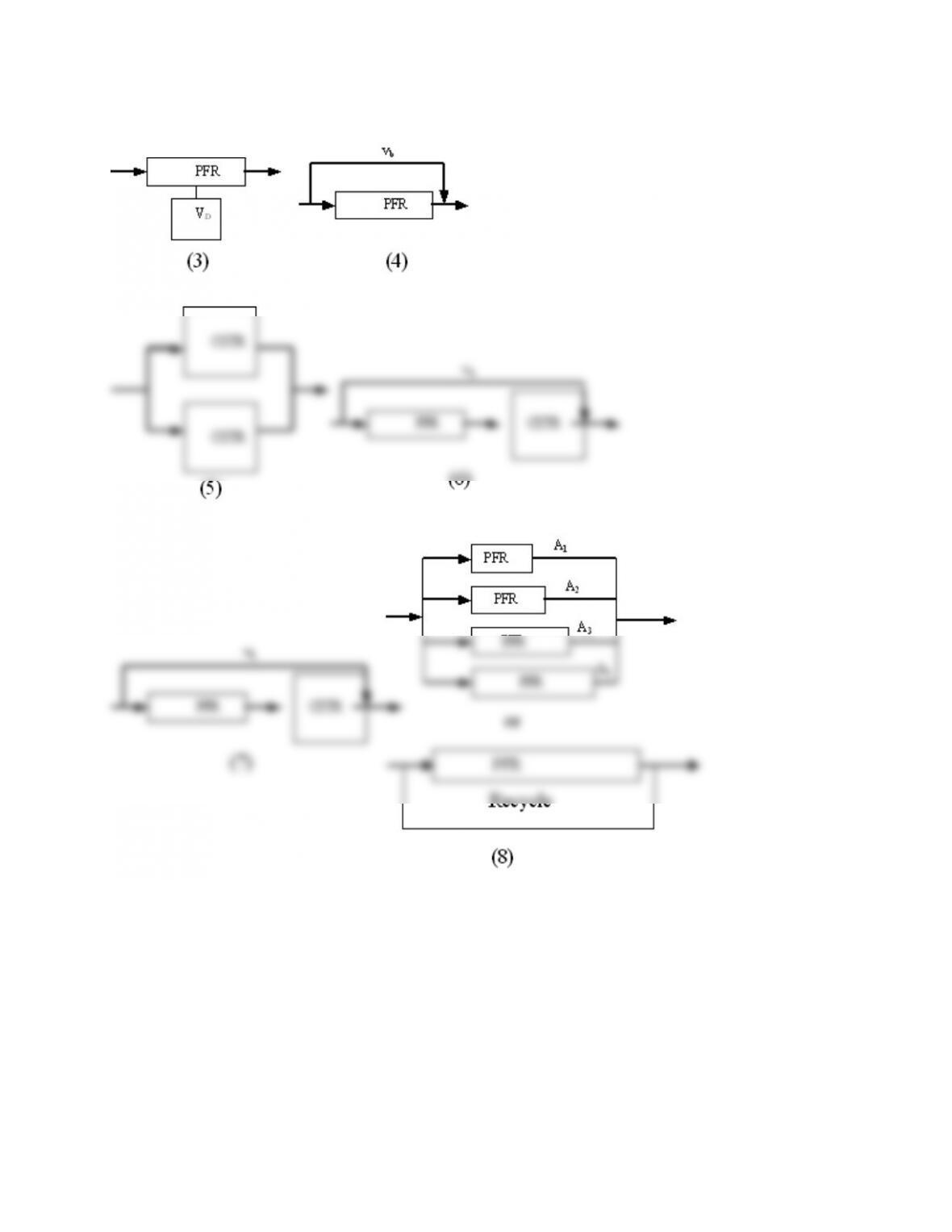
P14-13 (c)
Tanks in series and 1st order reaction with k=0.1min-1