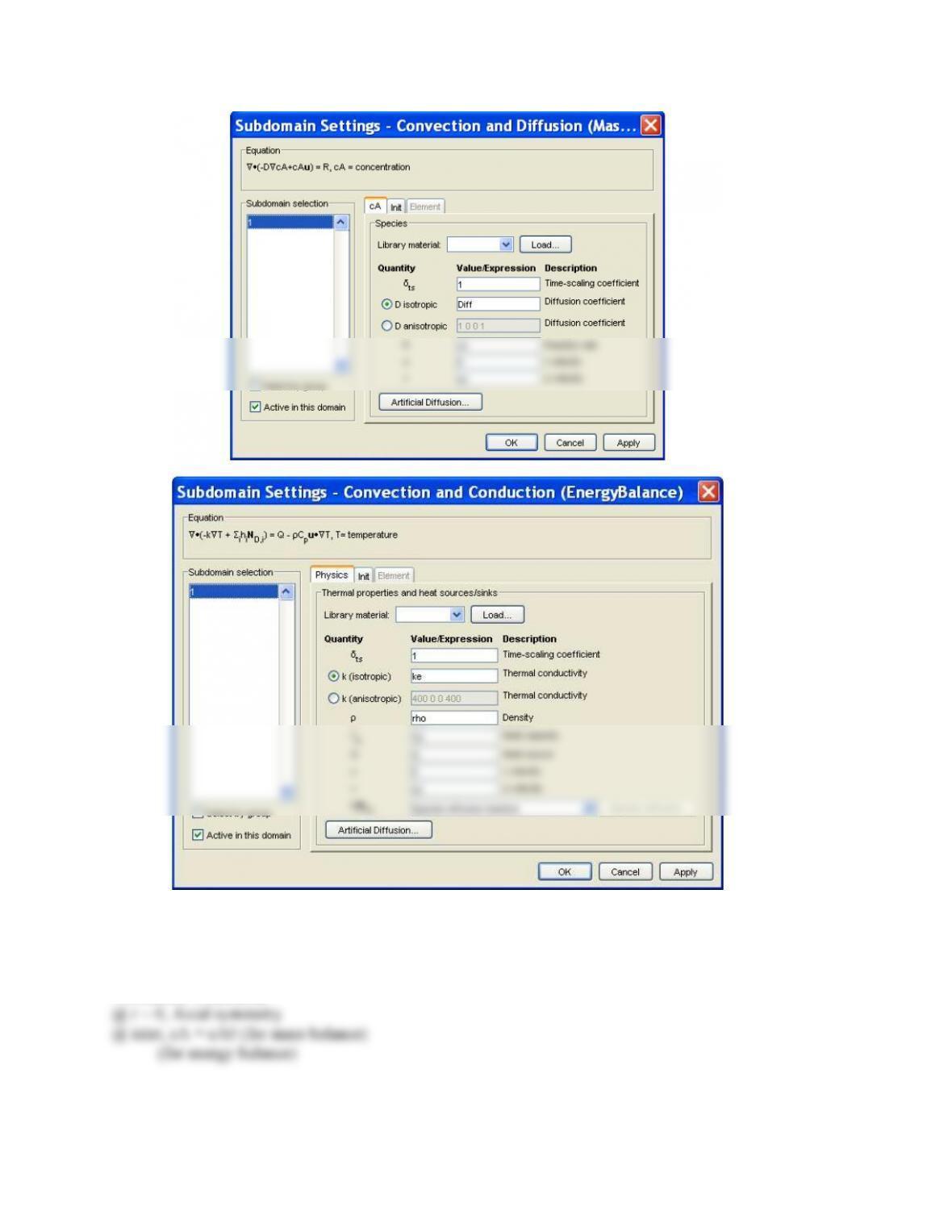
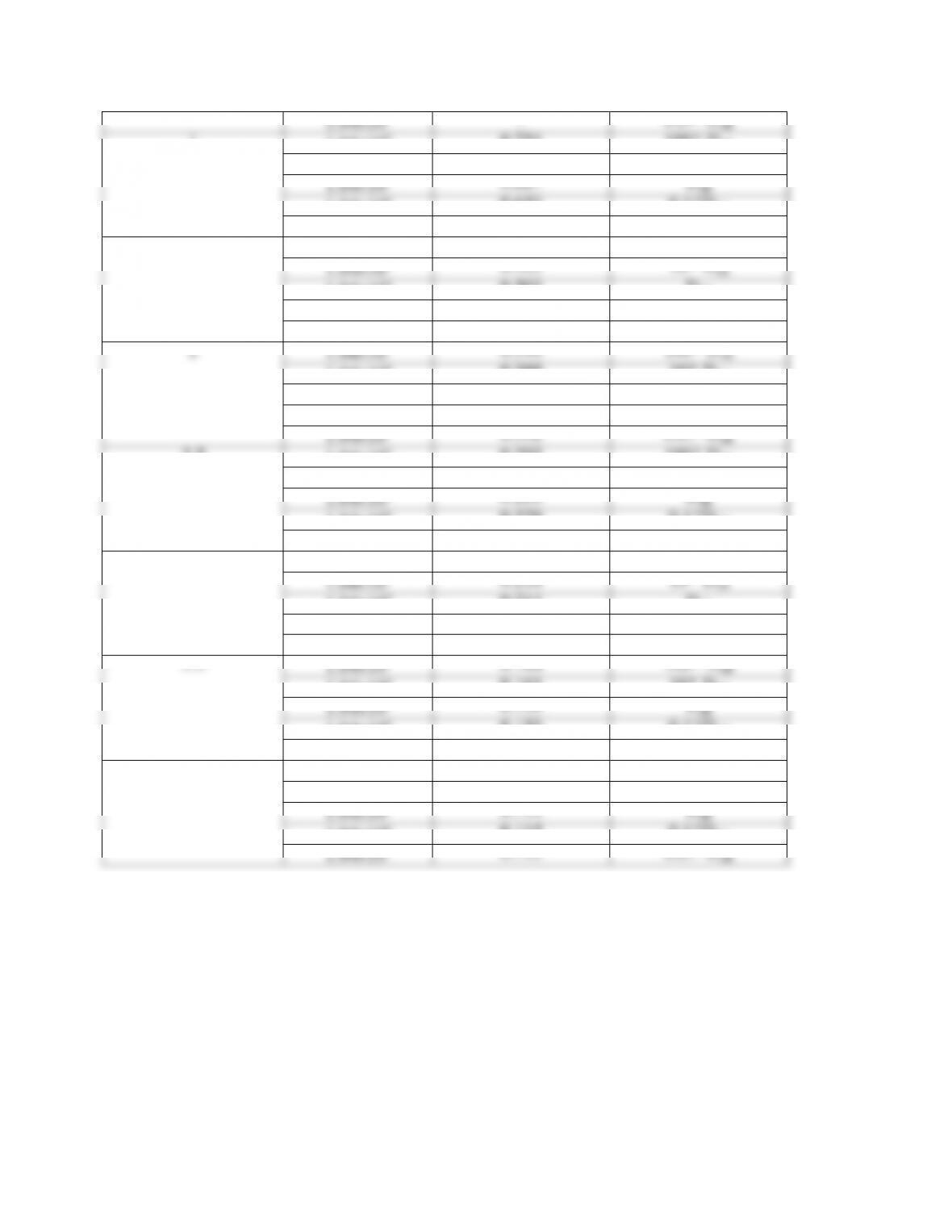
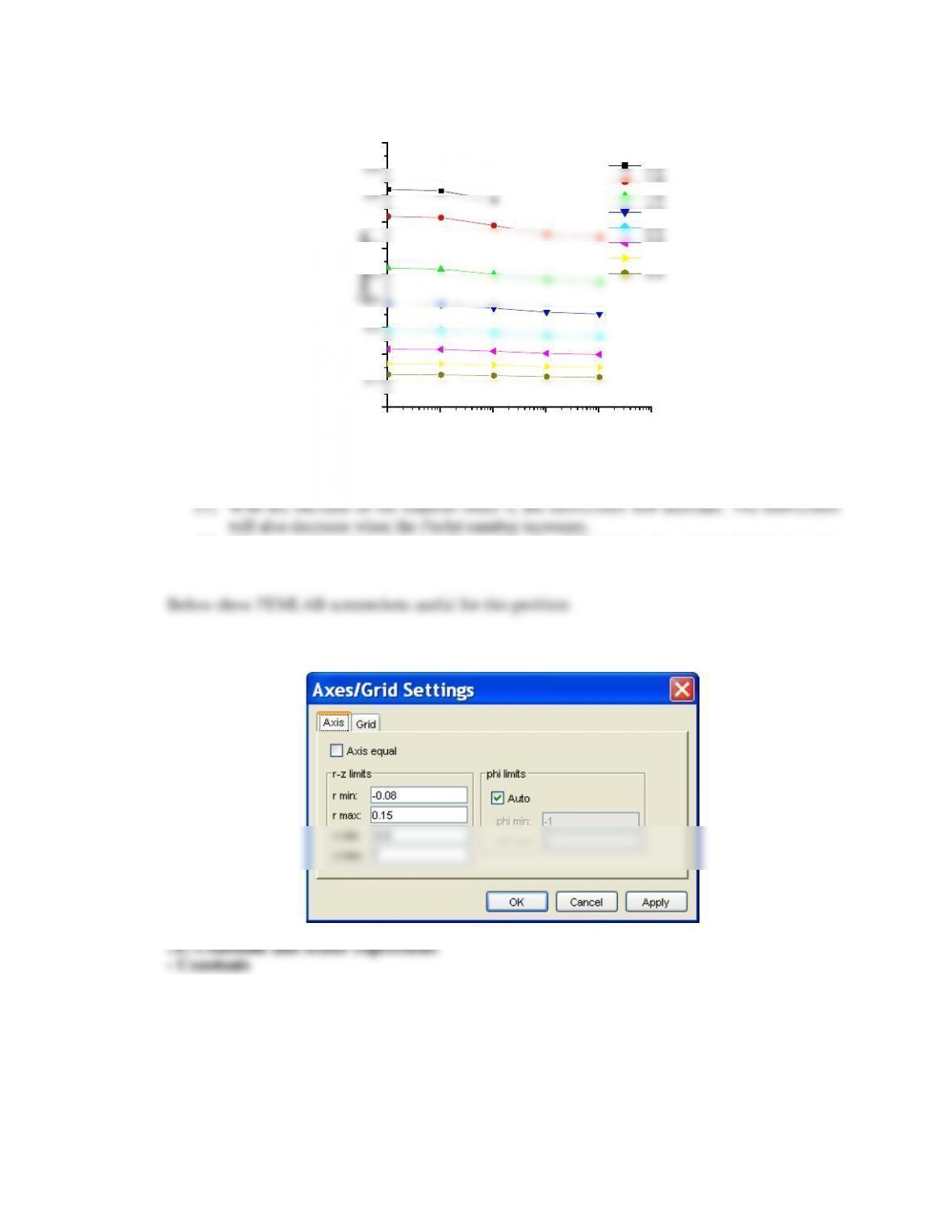
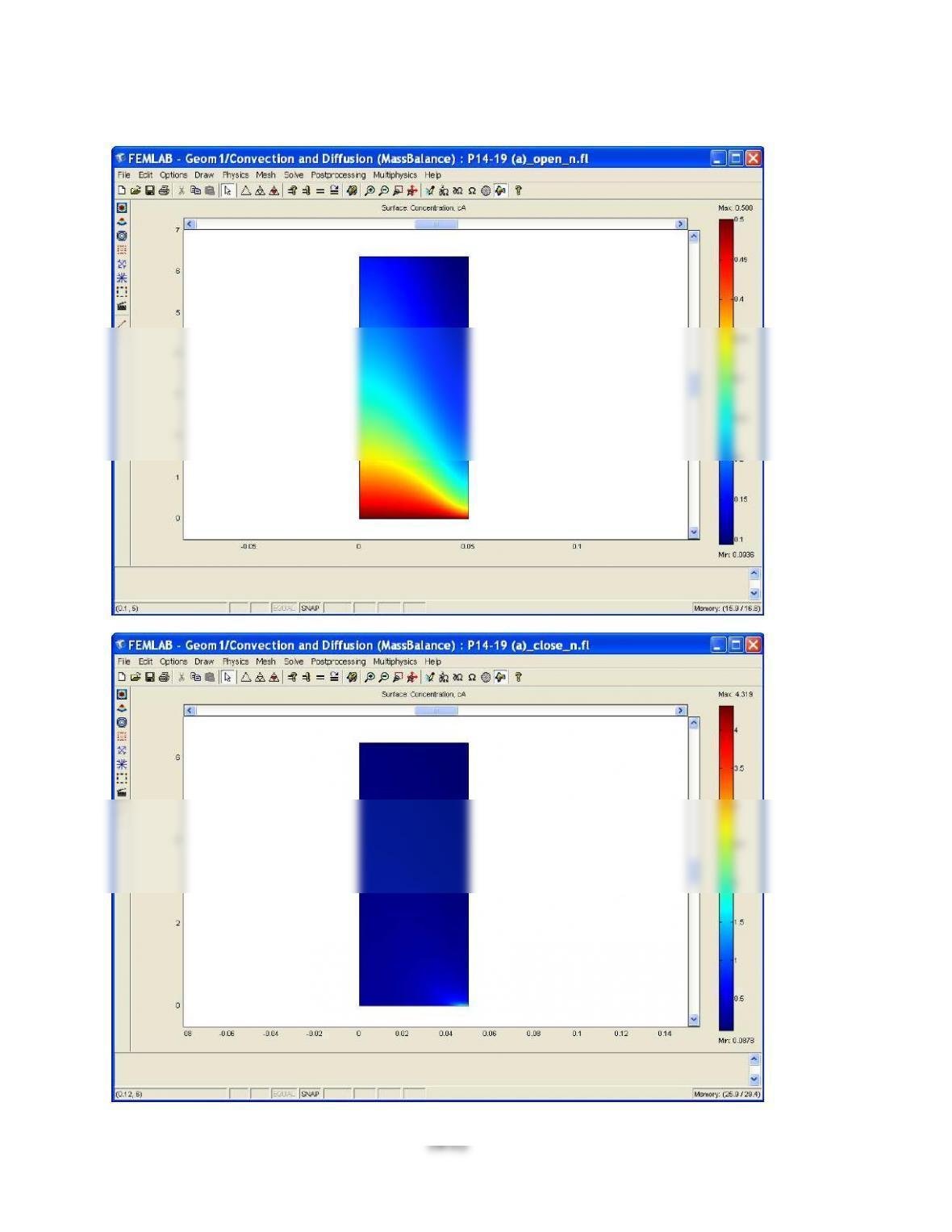
A parametric study of the numerical solutions of Eq.(14.51) and the enthalpy balance would give
the “exact” conditions for a minimum in the concentration.
A decrease in the thermal conductivity of the reaction mixtures determines an increase of the bulk
fluid temperature and can determine a minimum in concentration.
Overall heat transfer coefficient increases:
For a given flux this determines less deviation from the isothermal case: no minimum
Overall heat transfer coefficient decreases:
For a given flux this determines more deviation from the isothermal case
and the possibility of a minimum.
The external heat transfer coefficient increases, this implies a lower wall temperature and a
resulting lower temperature in the reacting mixture and less deviation from the isothermal case:
no minimum
The external heat transfer coefficient increases, this implies an increase in the wall temperature
and a resulting higher temperature in the reacting mixture that can determine a minimum in the
concentration.
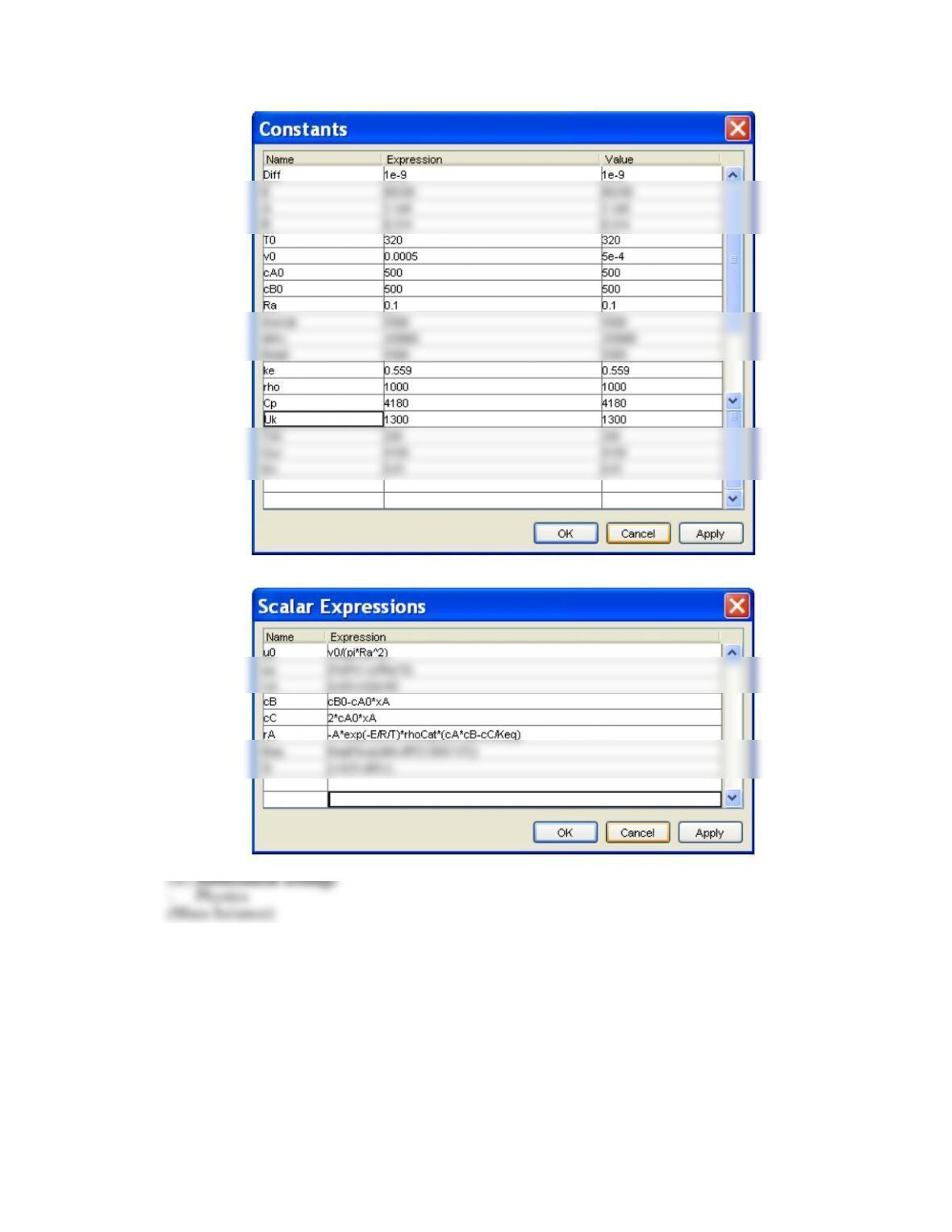
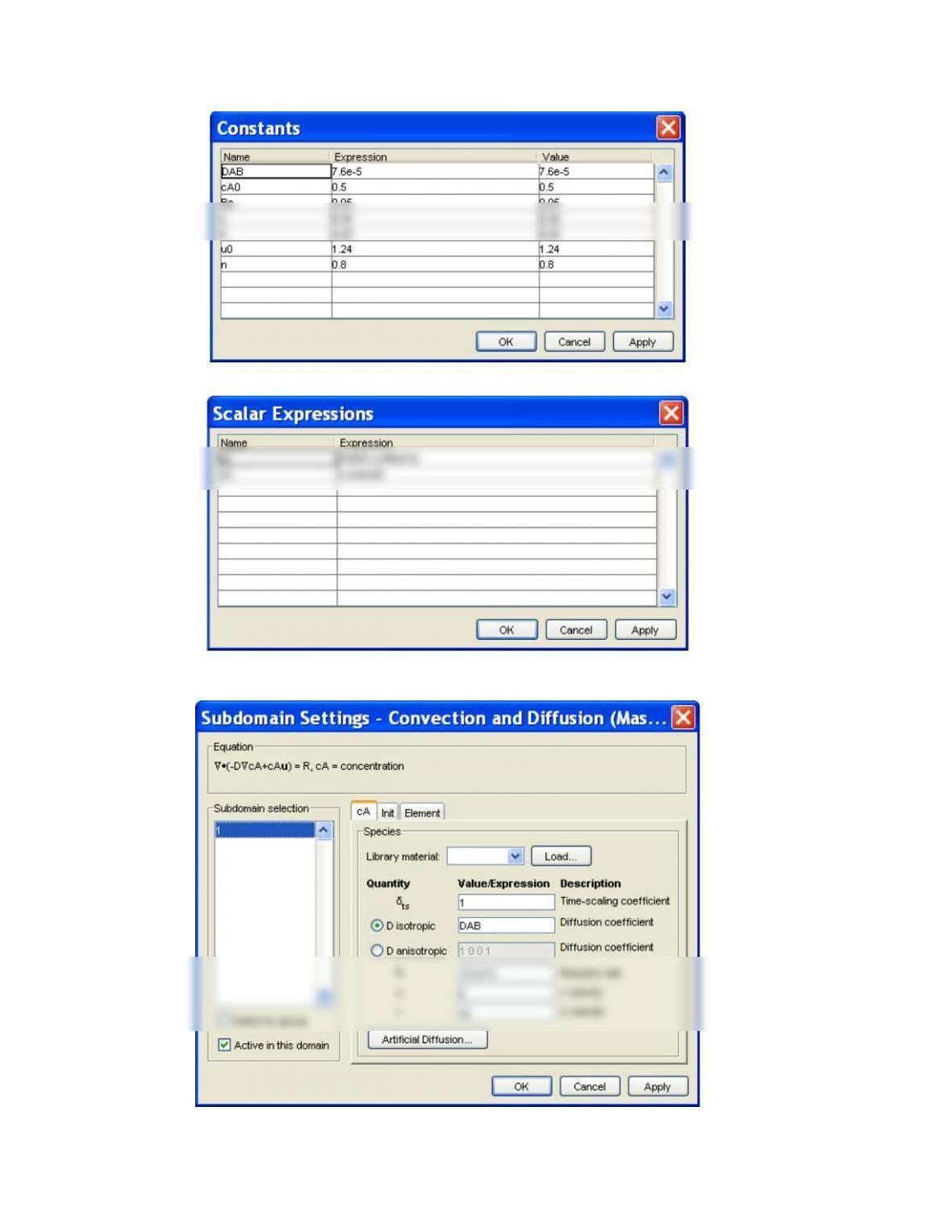
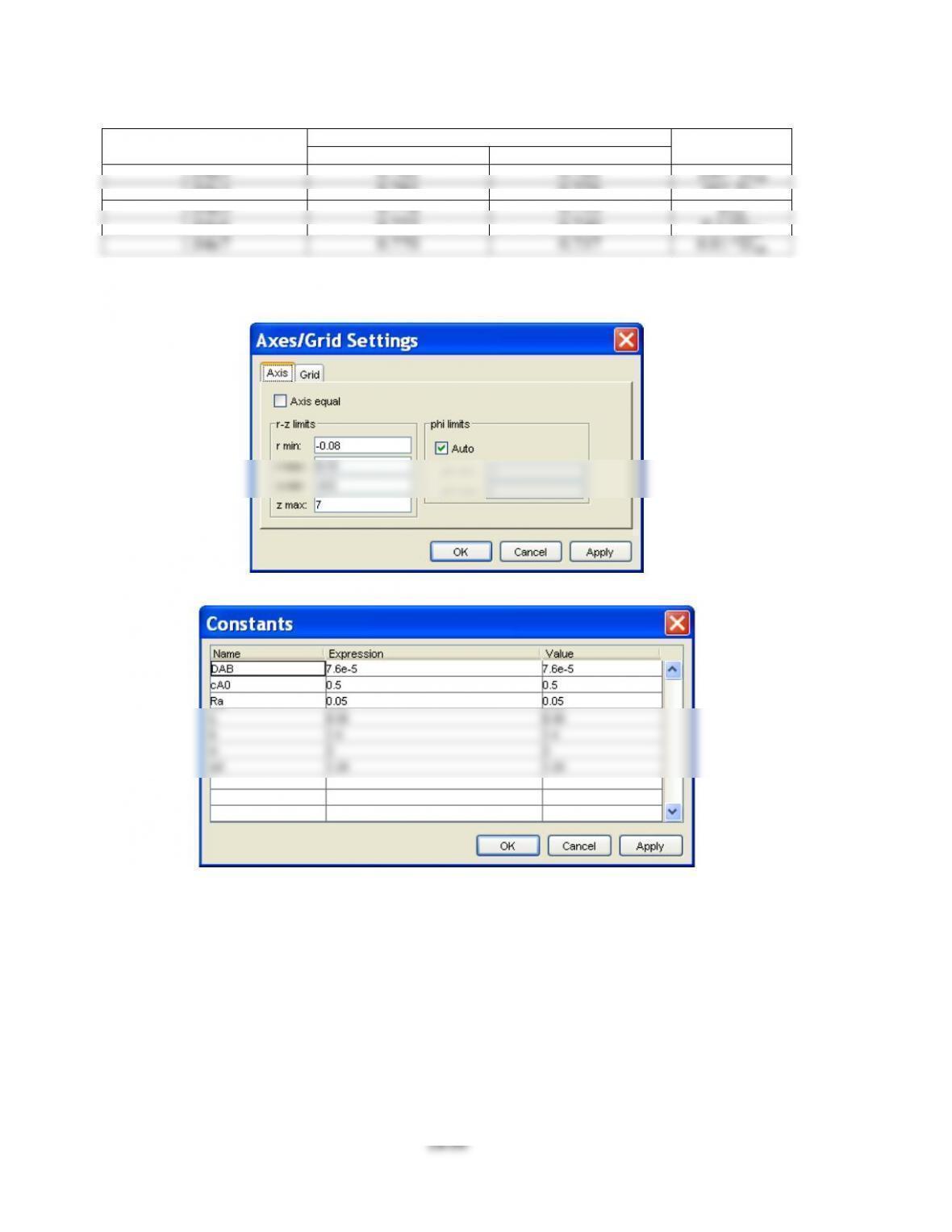
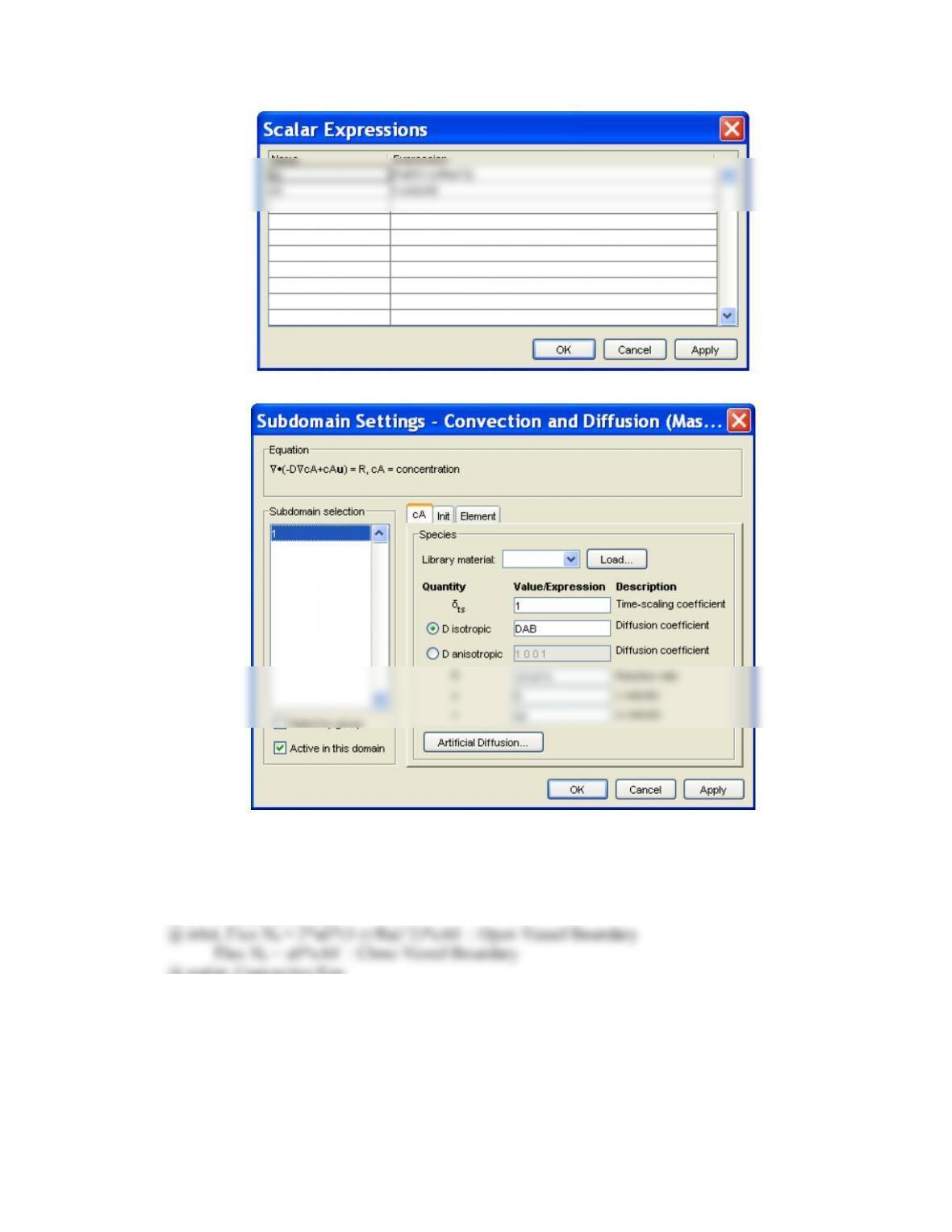
(2 ) Constants and scalar expressions
- Constants