14-13
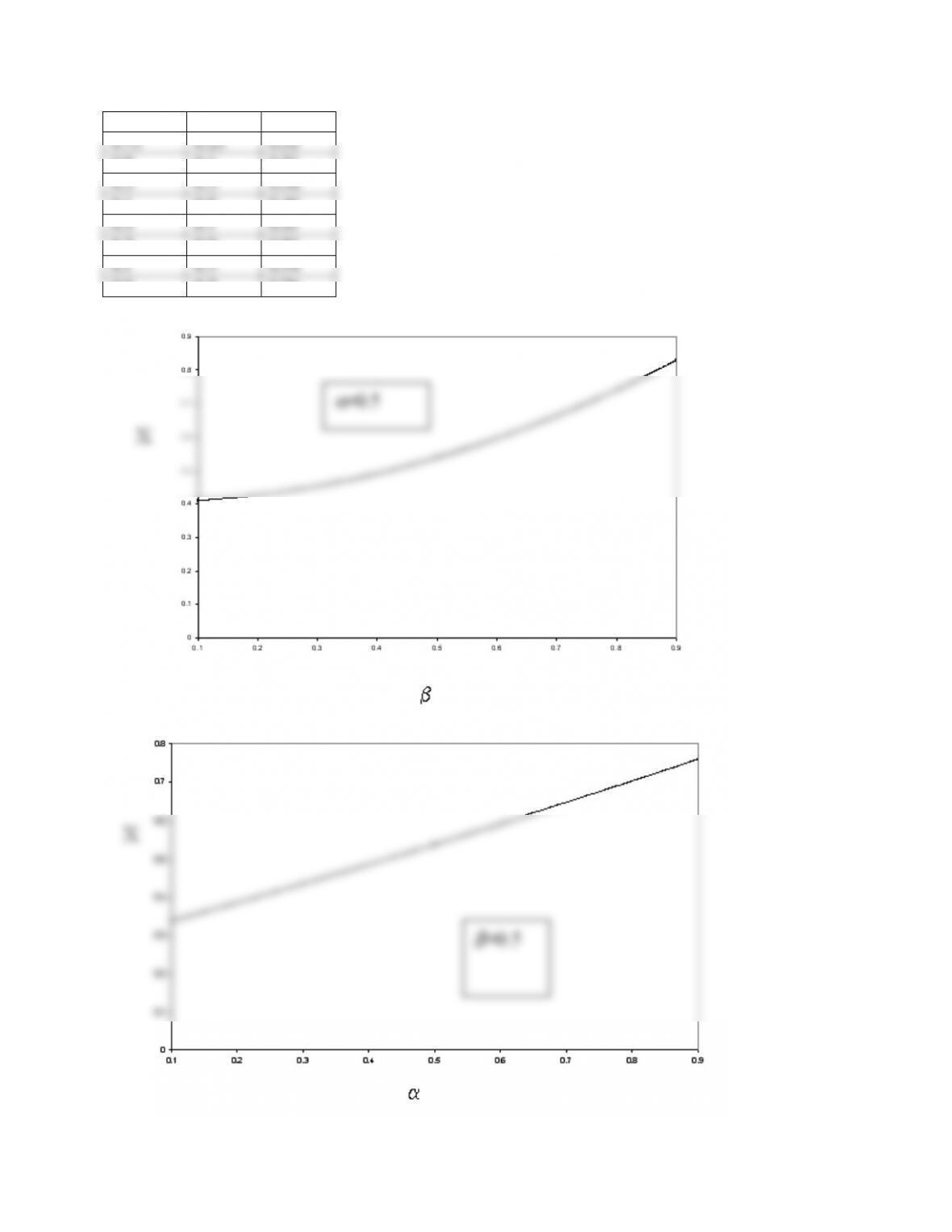
Given the interchange flowrate, the conversion is increased with the increasing of the volume of
the highly agitated reactor. Given the volume of the highly agitated reactor, the conversion is
increased with the increasing of the interchange.
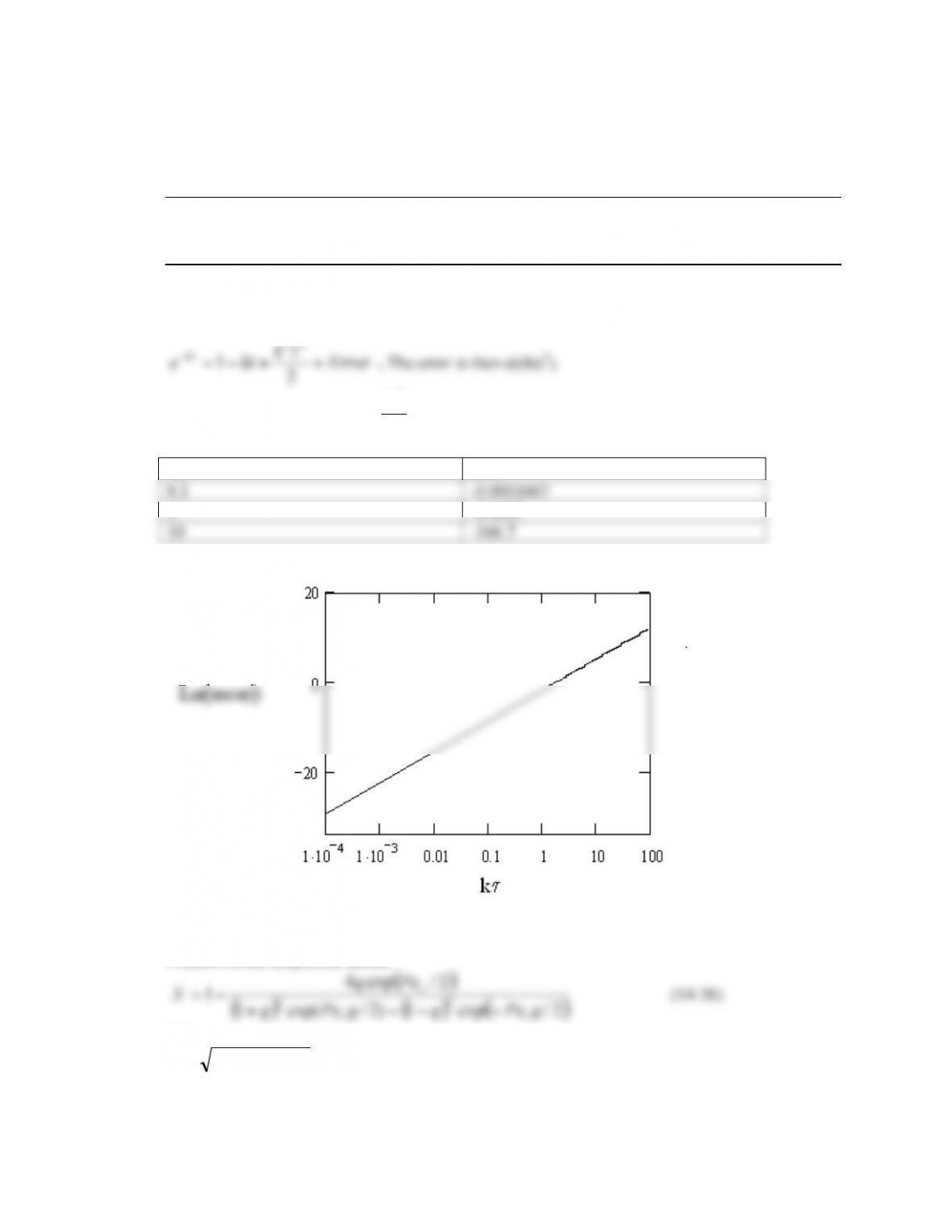
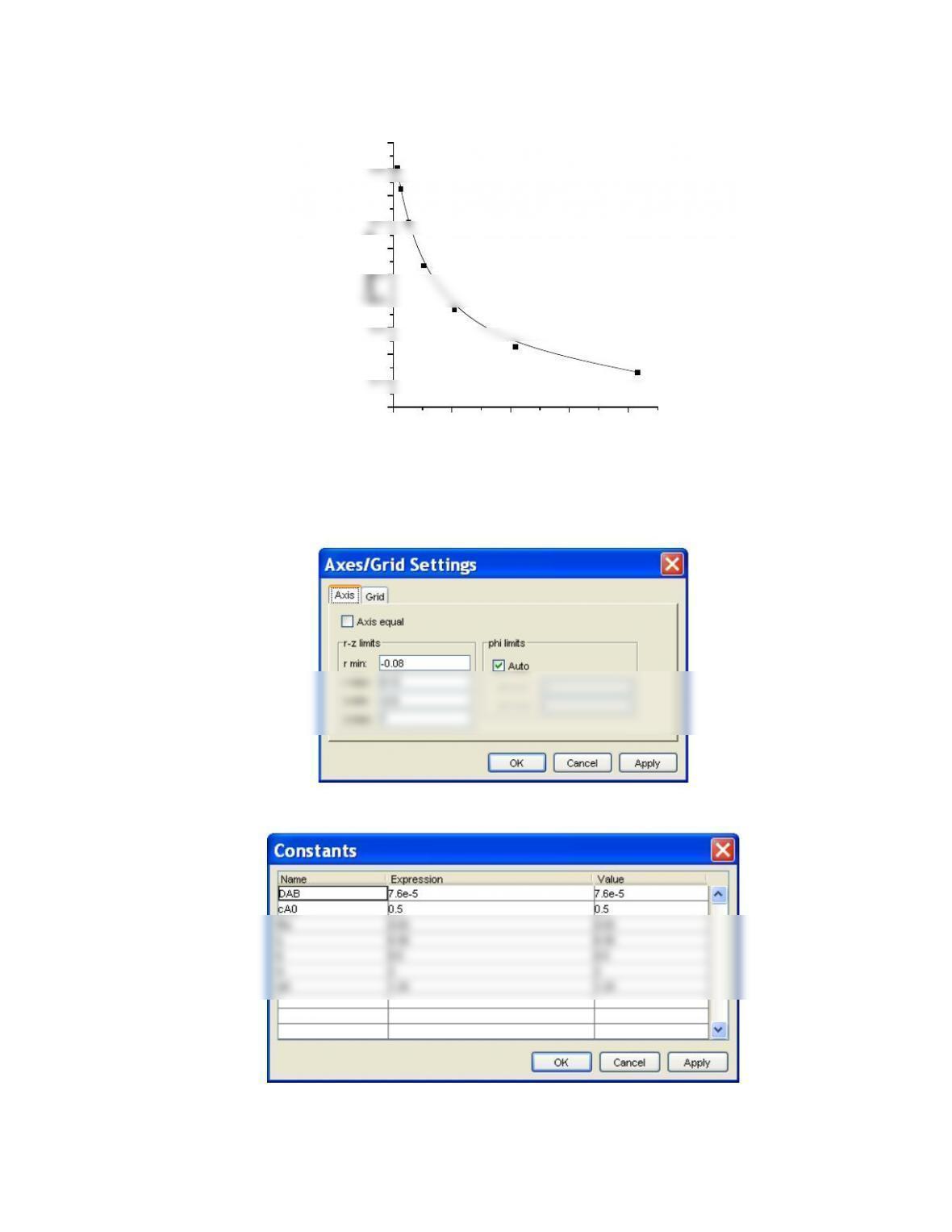
The correlations between Re and Da show what flow conditions (characterised by Re) give the
greatest or smallest Da and hence dispersion.
To minimise dispersion a Re number of ~10-20 gives the lowest value for Da. Because
,
May be a good approximation if CA does not change very much with time, i.e. A is in excess, in
which case CAo should not be divided by anything.
Linearizing the non 1st order reactions may give significantly inaccurate results using Equation
The curves in Fig.14.3 represent the residence time distributions for the Tanks in Series model as
function of the number of reactors.
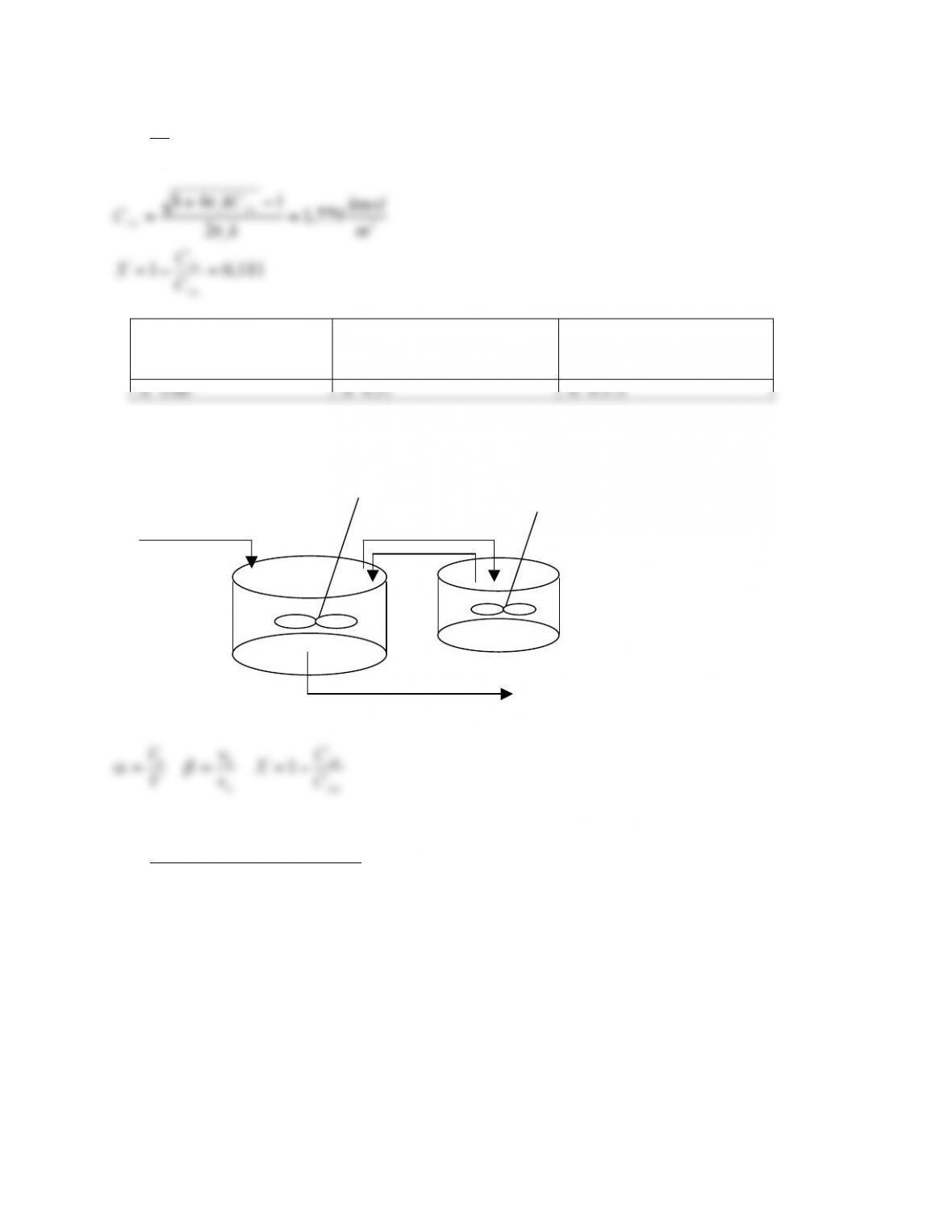
Given a CSTR of volume 1 (V=V1), we divide the CSTR in two CSTRs (V2=1/2). The mean
residence time is unchanged (V/F) but the molecules going out of the second reactor will be
delayed by the time (distribution) that occur to pass the first reactor (n=2, shift of the maximum).
In the limit of infinite division (Vn=0), so in the single CSTRs the residence time goes to zero for
all the molecules (zero variance), but their summation is the mean residence time (n=∞, PFR
the real reactor for less than time t shows a step up from zero when flow leaves from the “faster”
reactor. This fraction is the fraction of flow in the “slower” reactor. When the flow leaves the
“slower” reactor this fraction becomes one.
The model in Fig. 14.1 is PFR and CSTR in parallel. The exit age distribution for the CSTR is a
Conversion