13-13
Explicit equations as entered by the user
[1] T = 350
[2] k1 = exp((5000/1.987)*(1/350-1/T))
[3] k2 = exp((1000/1.987)*(1/350-1/T))
[4] E1 = -2.104*t^4+4.167*t^3-1.596*t^2+0.353*t-0.004
[5] E2 = -2.104*t^4+17.037*t^3-50.247*t^2+62.964*t-27.402
[6] rc = k1*ca*cb
[7] k3 = exp((9000/1.987)*(1/350-1/T))
[9] re = k3*cb*cd
[10] E = if(t<=1.26)then(E1)else(E2)
[11] rb = -k1*ca*cb-k3*cb*cd
[12] Scd = cc/(cd+.000000001)
[13] Sde = cd/(ce+.000000000001)
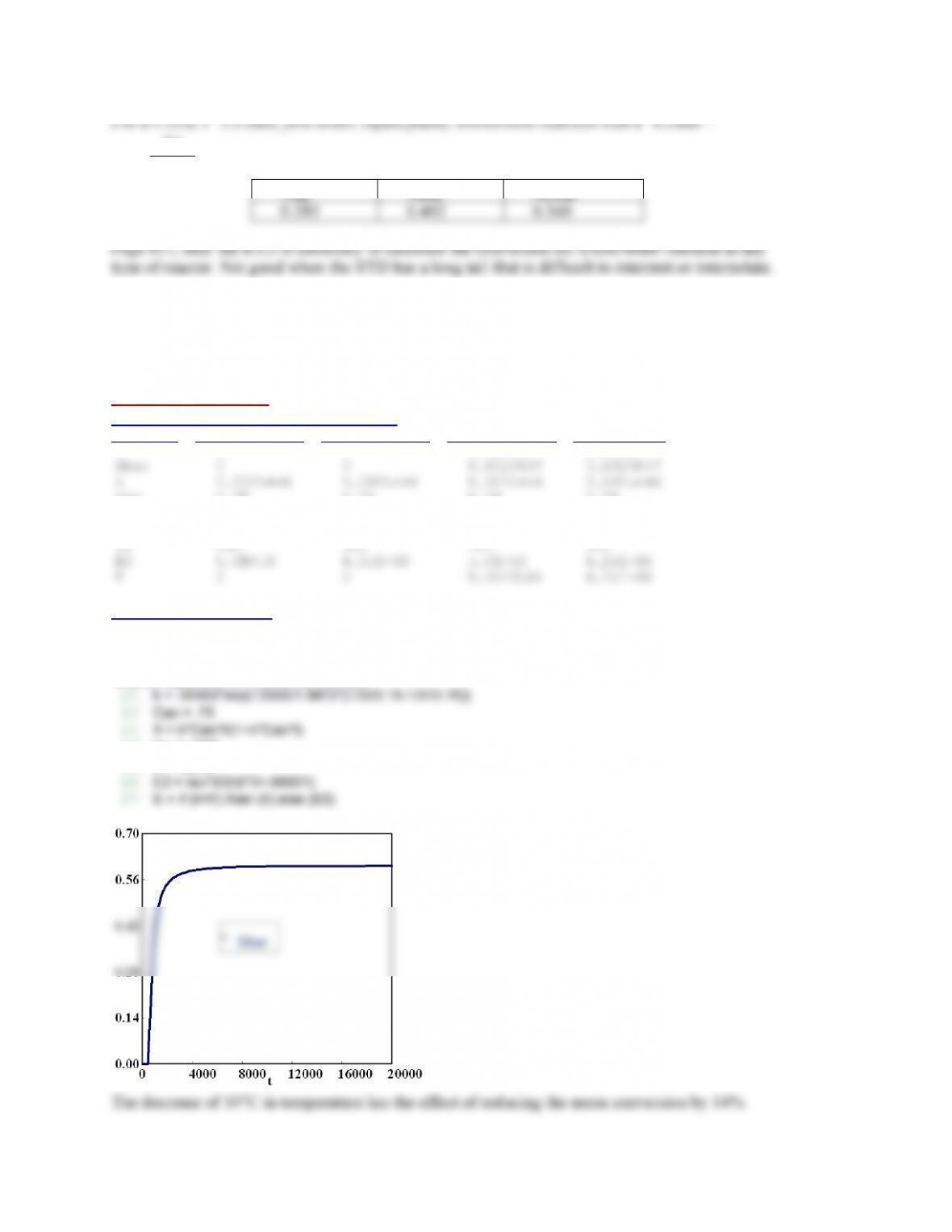
If the temperature is raised, the conversion of A increases. The selectivity Sc/d increases with
temperature and Sd/e decreases with increasing temperature
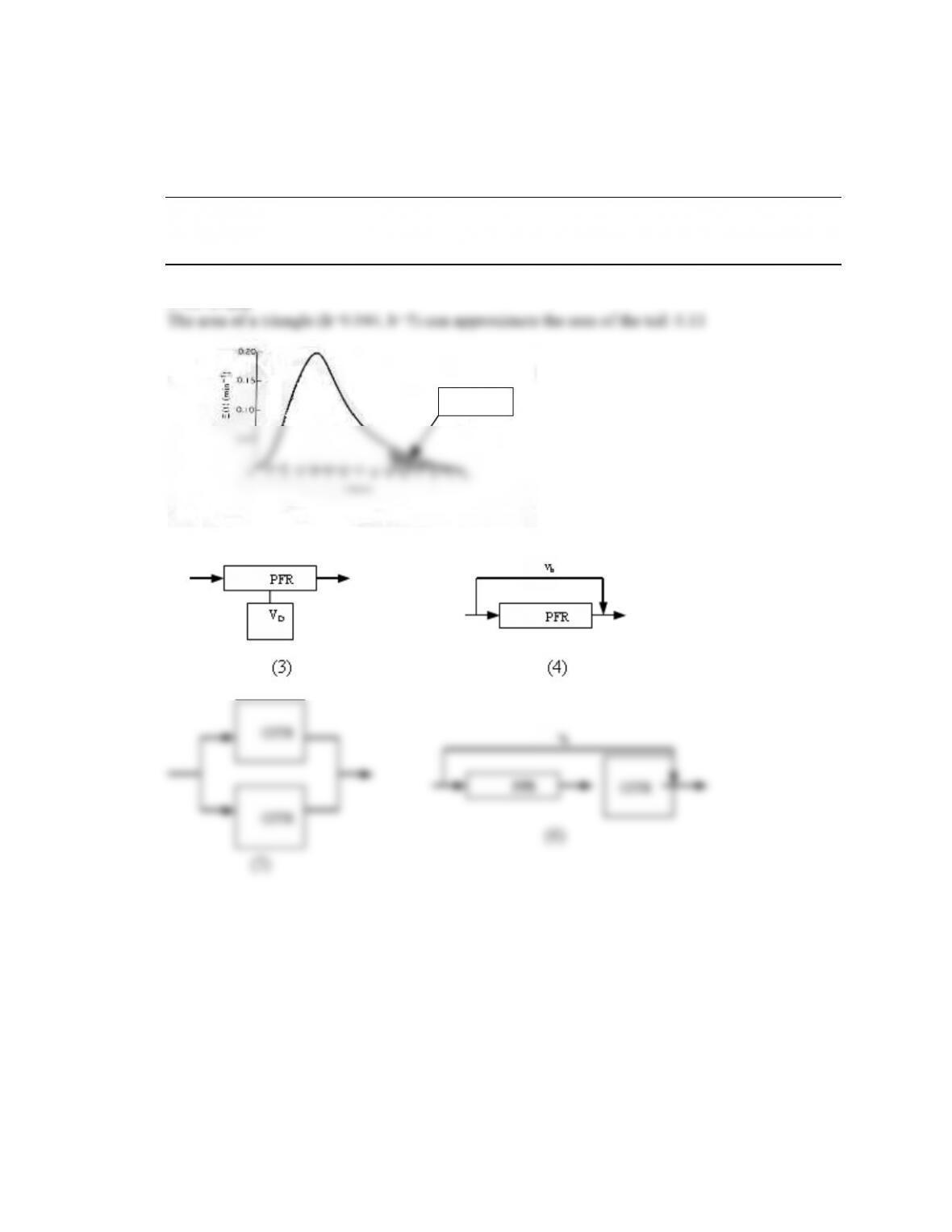
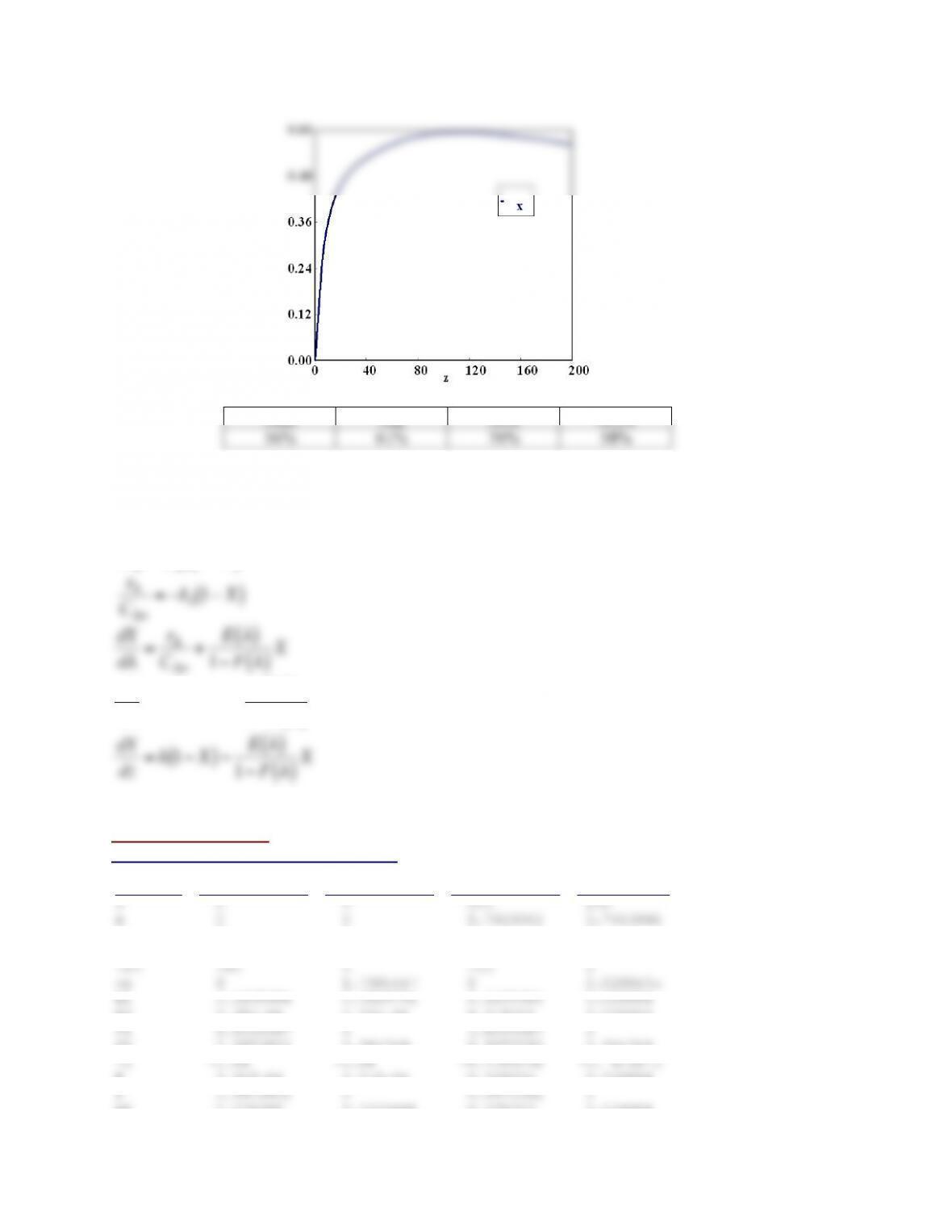
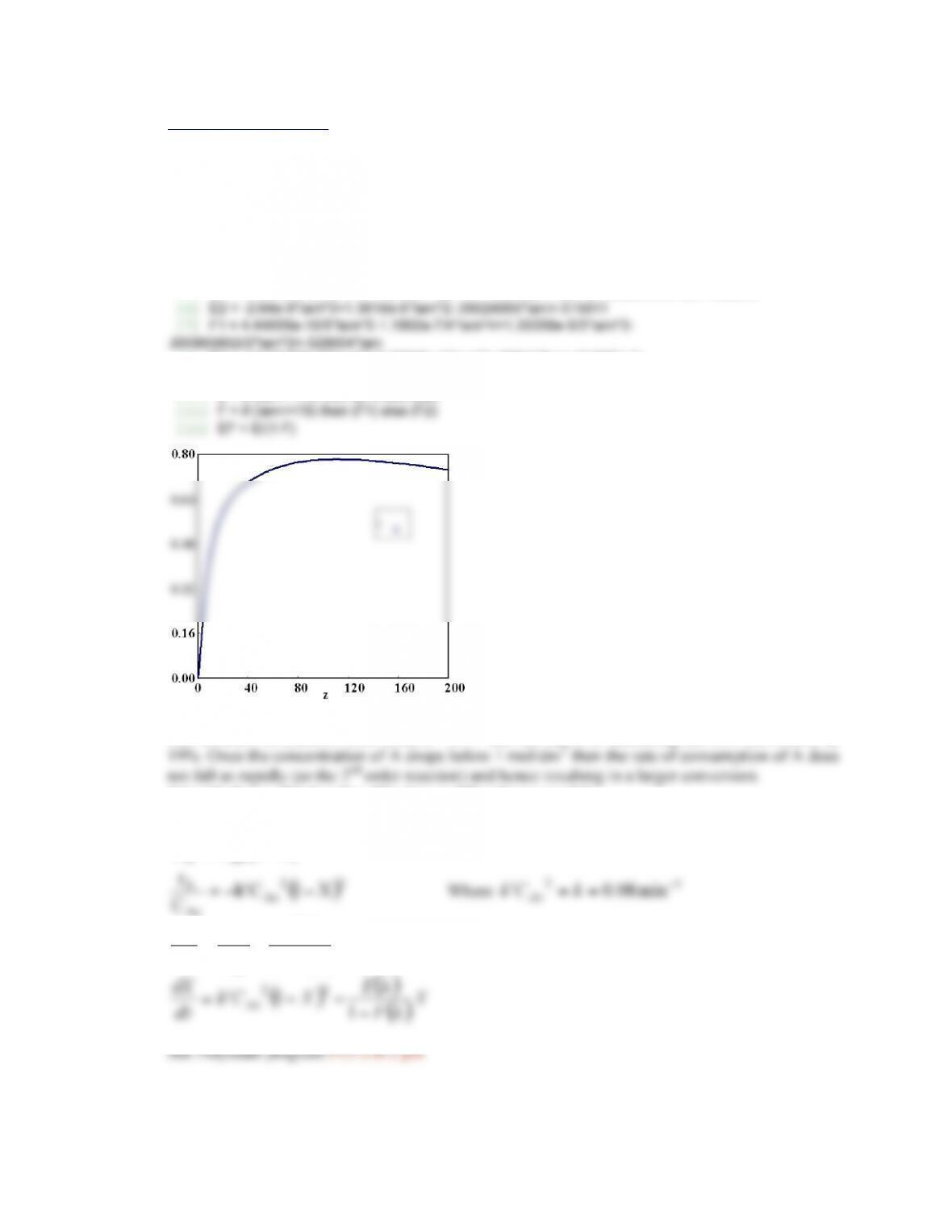
Bimodal RTD
See Polymath program P13-2-i-2.pol
Variable initial value minimal value maximal value final value
z 0 0 6 6
ca 1 0.2660482 1 0.2660482
cb 1 0.5350642 1 0.5352659
cc 0 0 0.2872257 0.2745726
F 0.99 -0.0033987 0.99 -0.0033987
cbo 1 1 1 1
cao 1 1 1 1
cco 0 0 0 0
k2 1 1 1 1
k1 1 1 1 1
rc 1 0.1424065 1 0.1424065
E2 6737.4446 0.0742397 6737.4446 925.46463
E3 0.00911 0.0061156 1.84445 1.84445
re 0 0 0.1694414 0.144103
E 0.00911 0.0061156 0.6288984 0.20909
EF 0.911 0.2083818 1.8694436 0.2083818
Scd 0 0 1.0856272 1.0198908