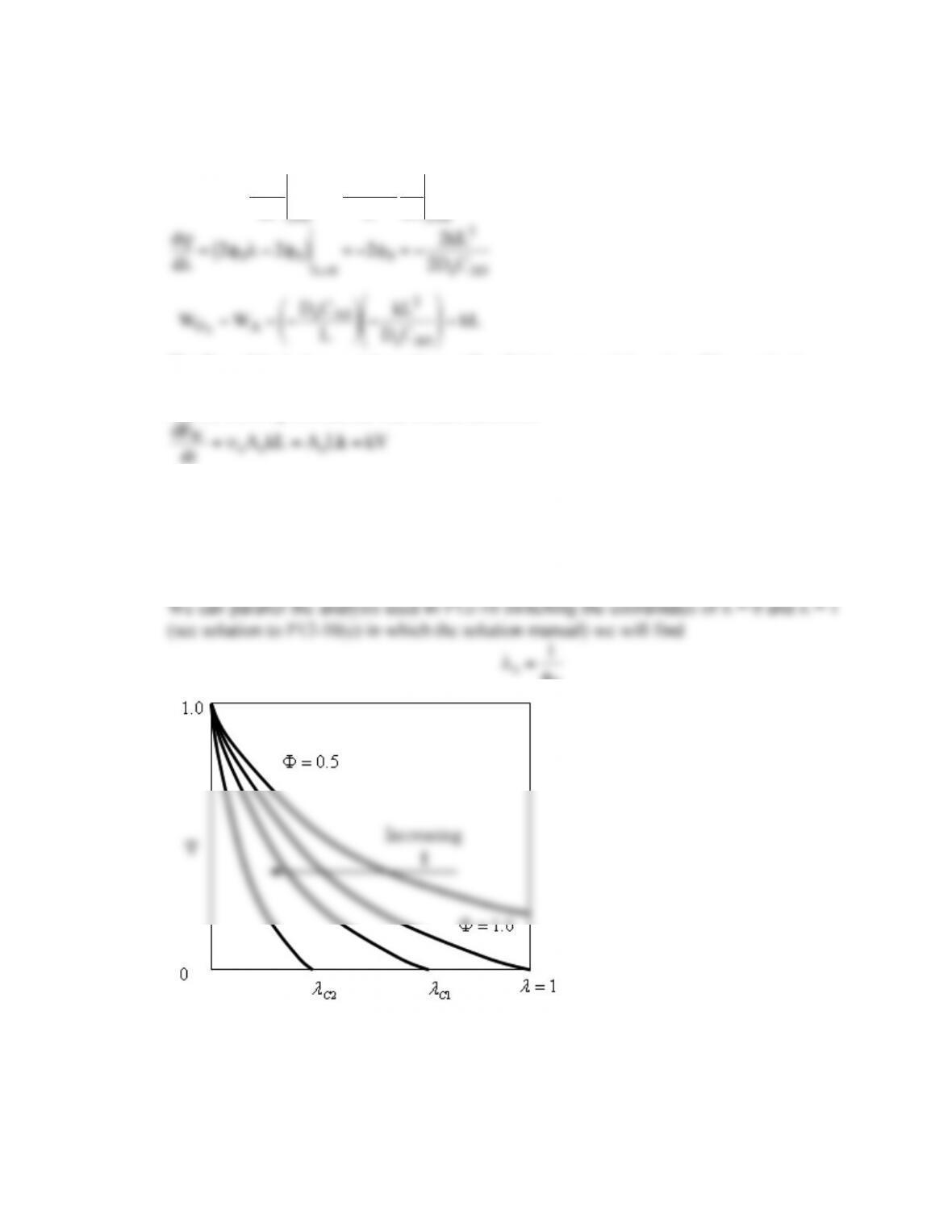
P12-2 (h)
The activation energy will be larger than that for diffusion control and hence the reaction is more
temperature sensitive. If the apparent reaction order is greater than one half, then the rate of reaction will be
less sensitive to concentration. If it is less than one half, the true order will be negative and the rate will
increase significantly at low concentration.
In example CDR12-1, the reactor is 5 m in diameter and 22 m high, whereas the reactor in CDR12-2 is
only 2 m3 in volume. The charge is much different. In CDR12-1 the charge is 100 kg/m3 and in CDR12-2 it
is only 3.9 kg/m3
P12-2 (k)
With the increase in temperature, the rate of reaction will increase. This will cause the slope of Ci/Ri vs.
1/m and, therefore, the resistance to decrease.
P12-3 (a) Yes
P12-3 (b)
All temperatures, FT0 = 10 mol/hr. The rate of reaction changes with flow rate and increases linearly with
temperature
P12-3 (d)
T < 367 K, FT0 = 1000 mol/hr, 5000 mol/hr.
T < 362 K, FT0 = 100 mol/hr.