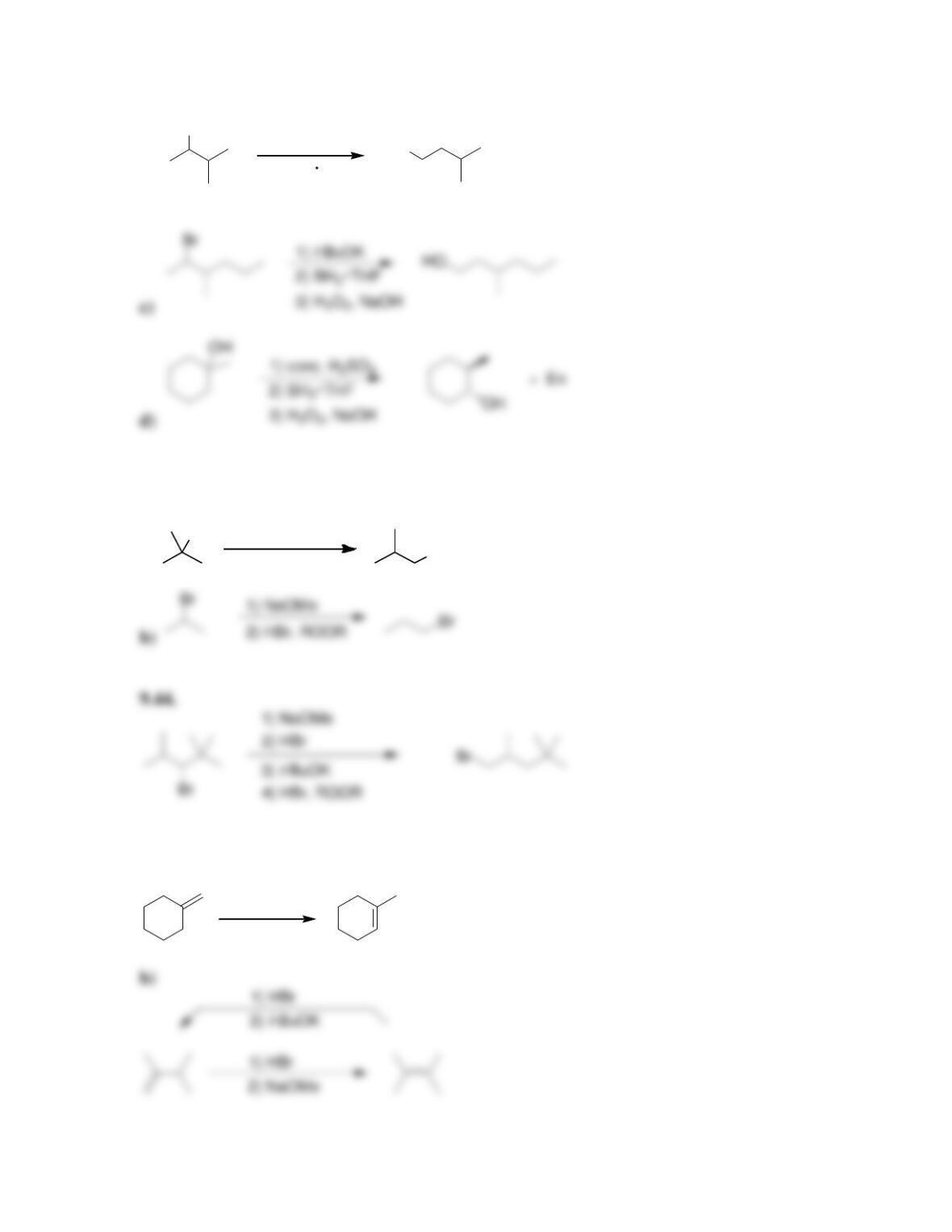
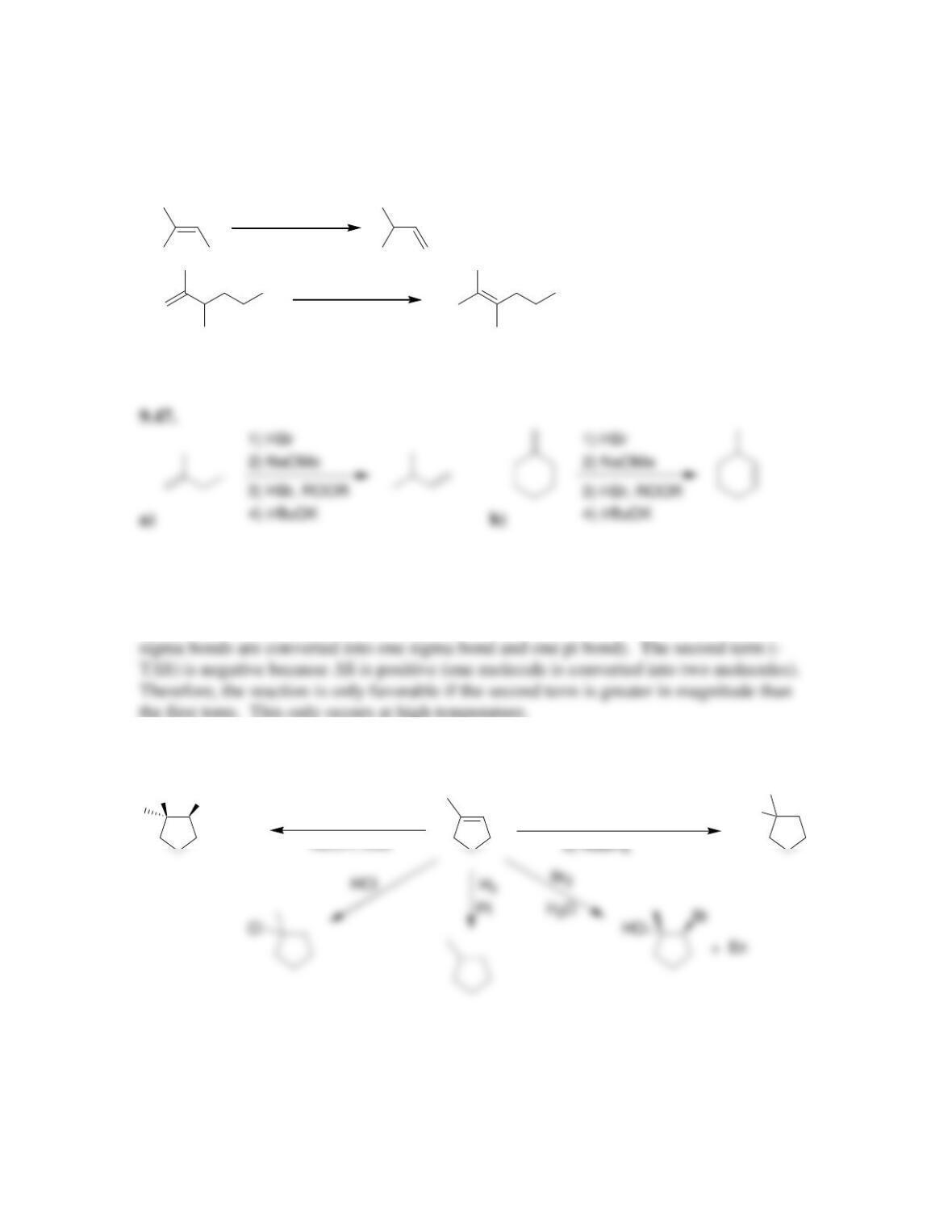
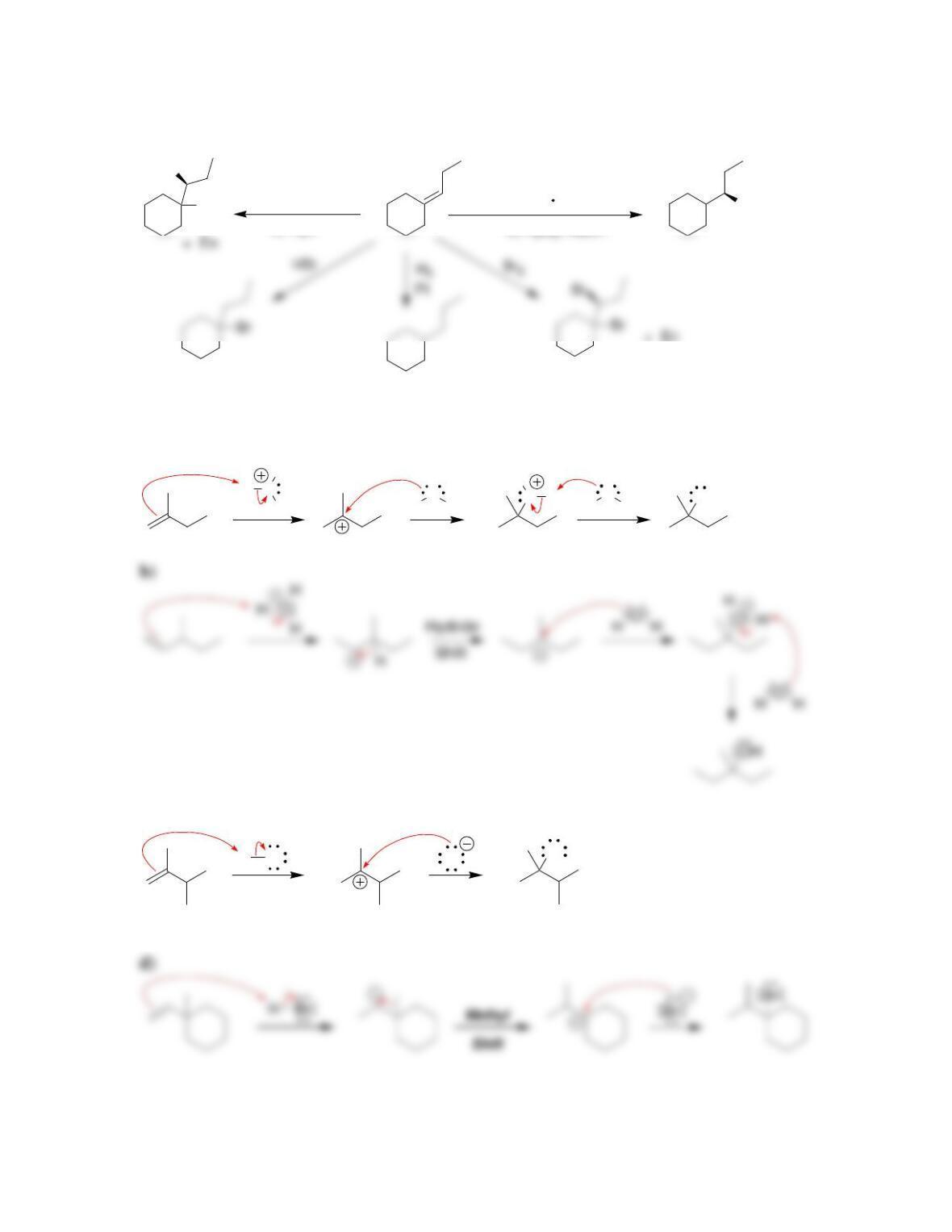
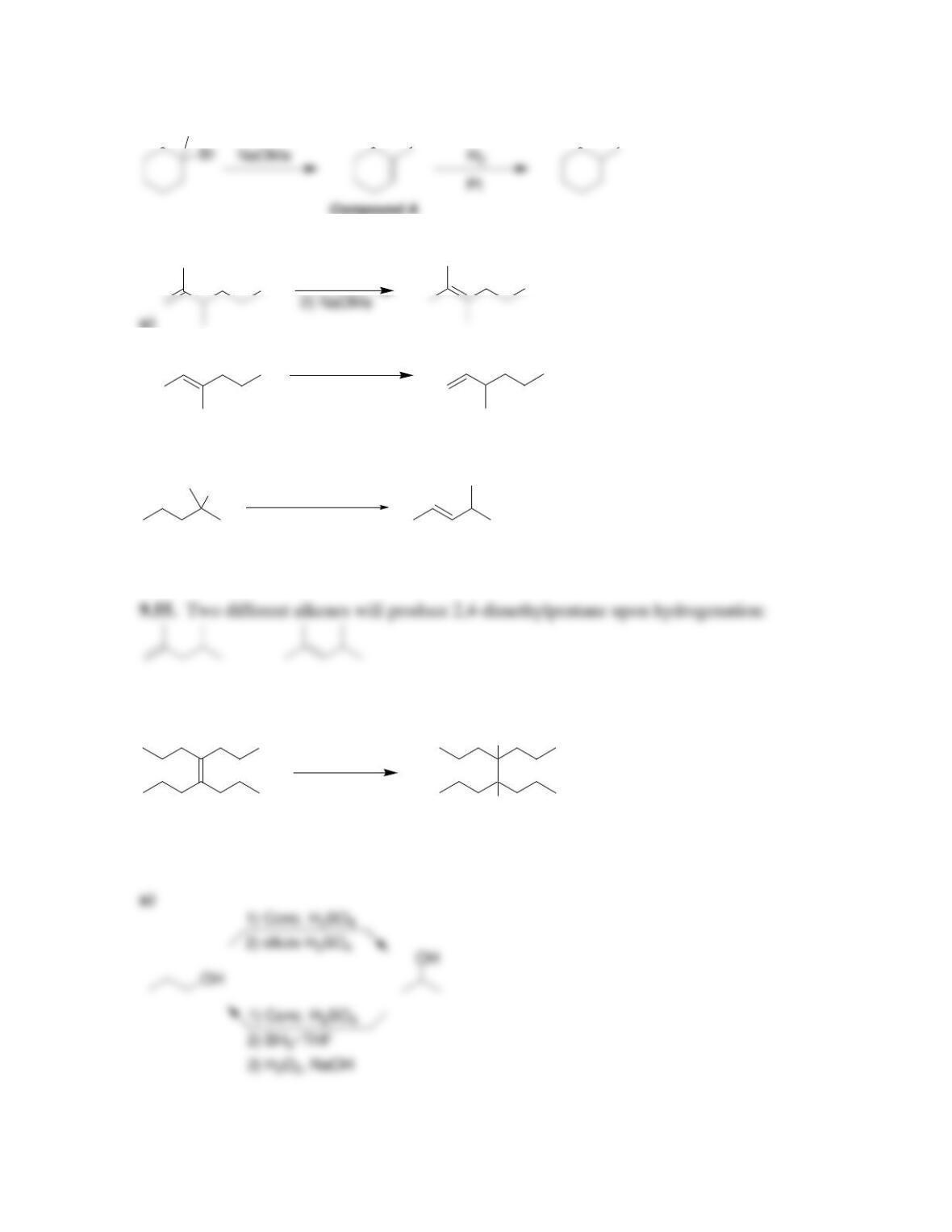
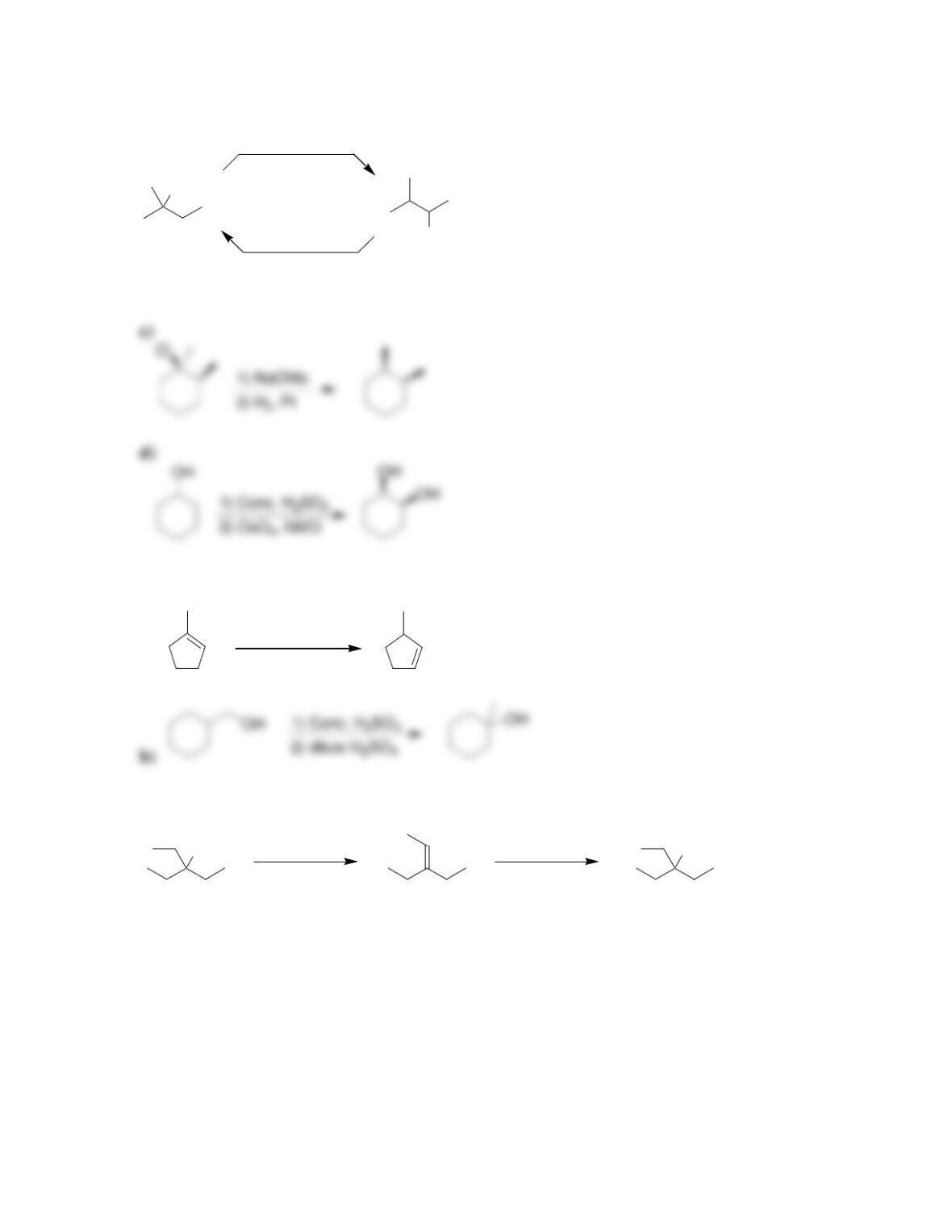
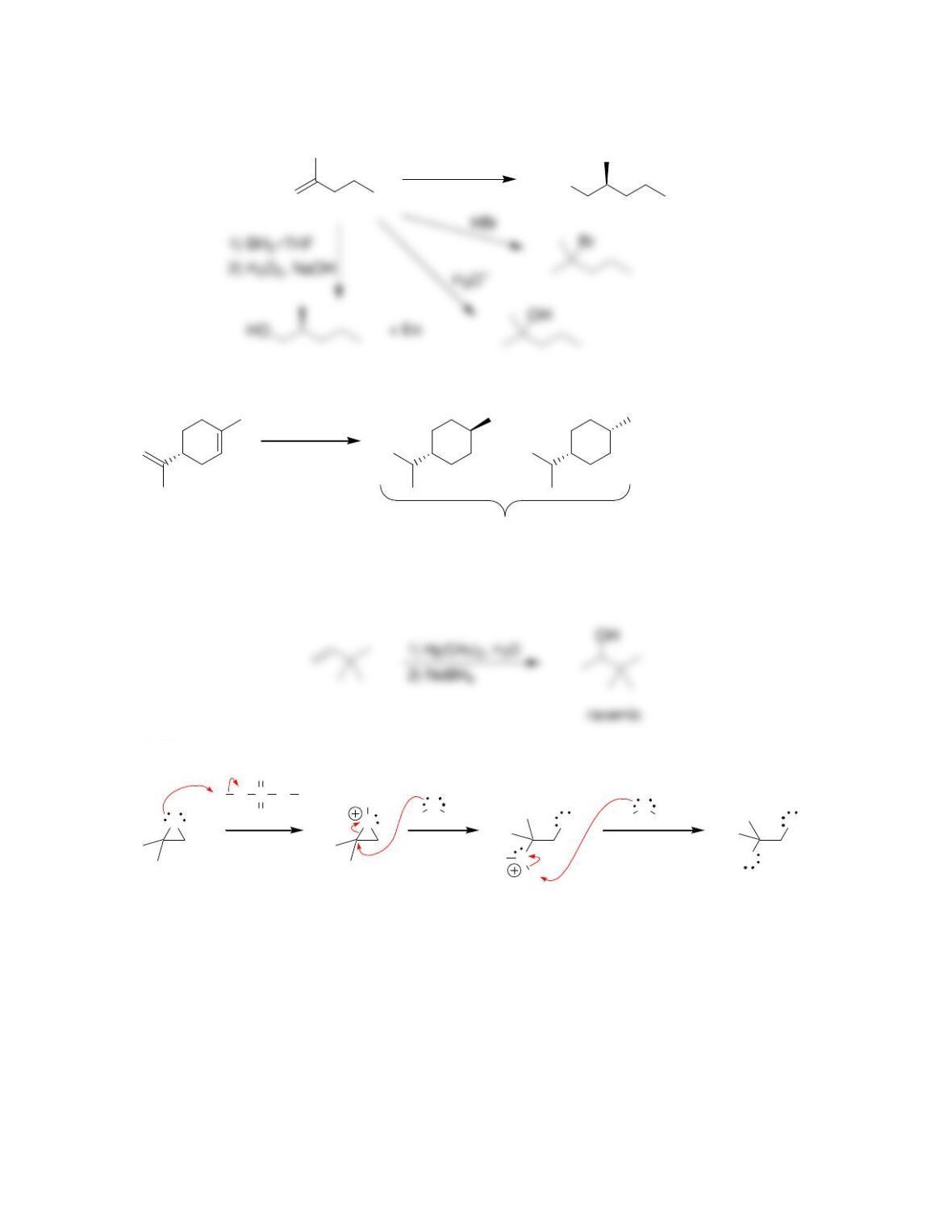
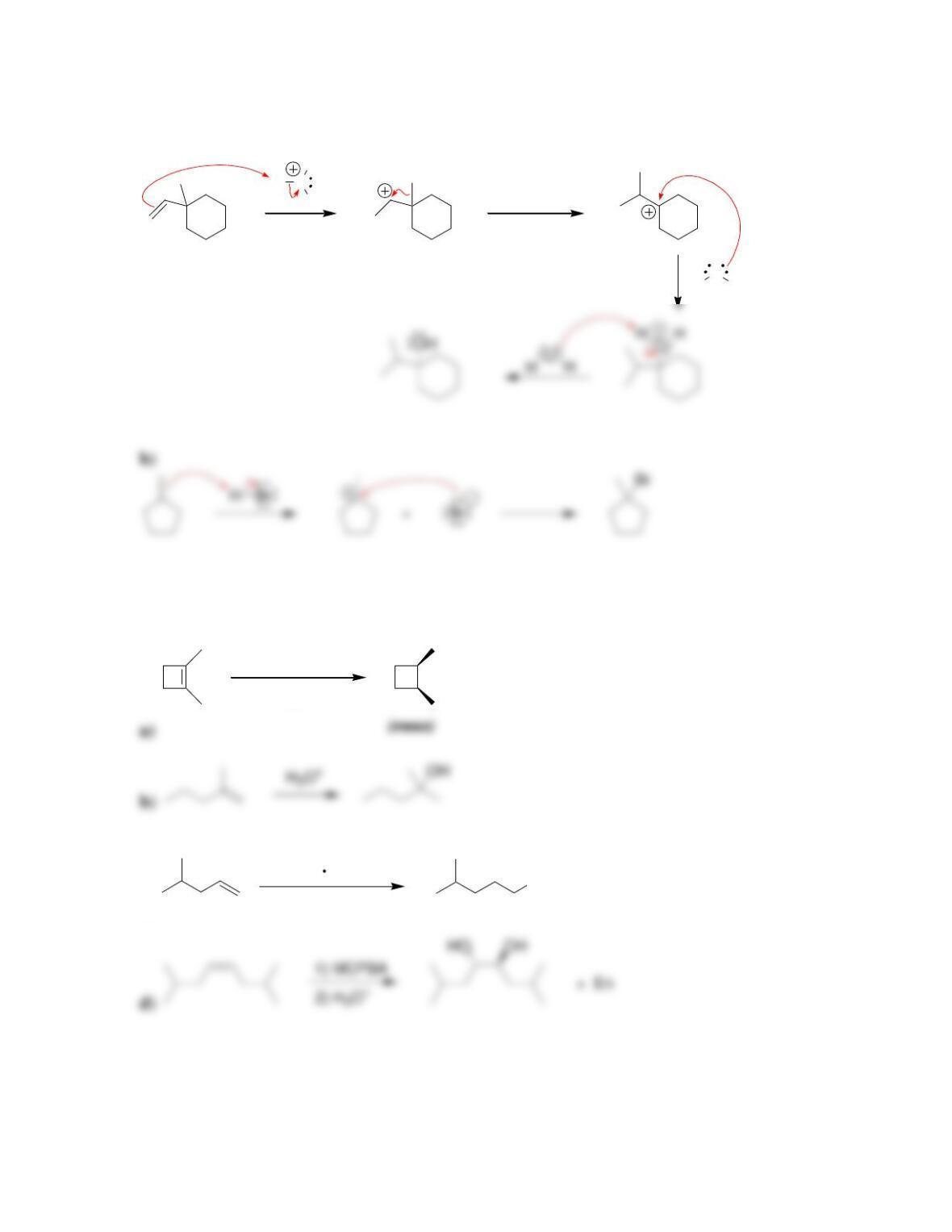
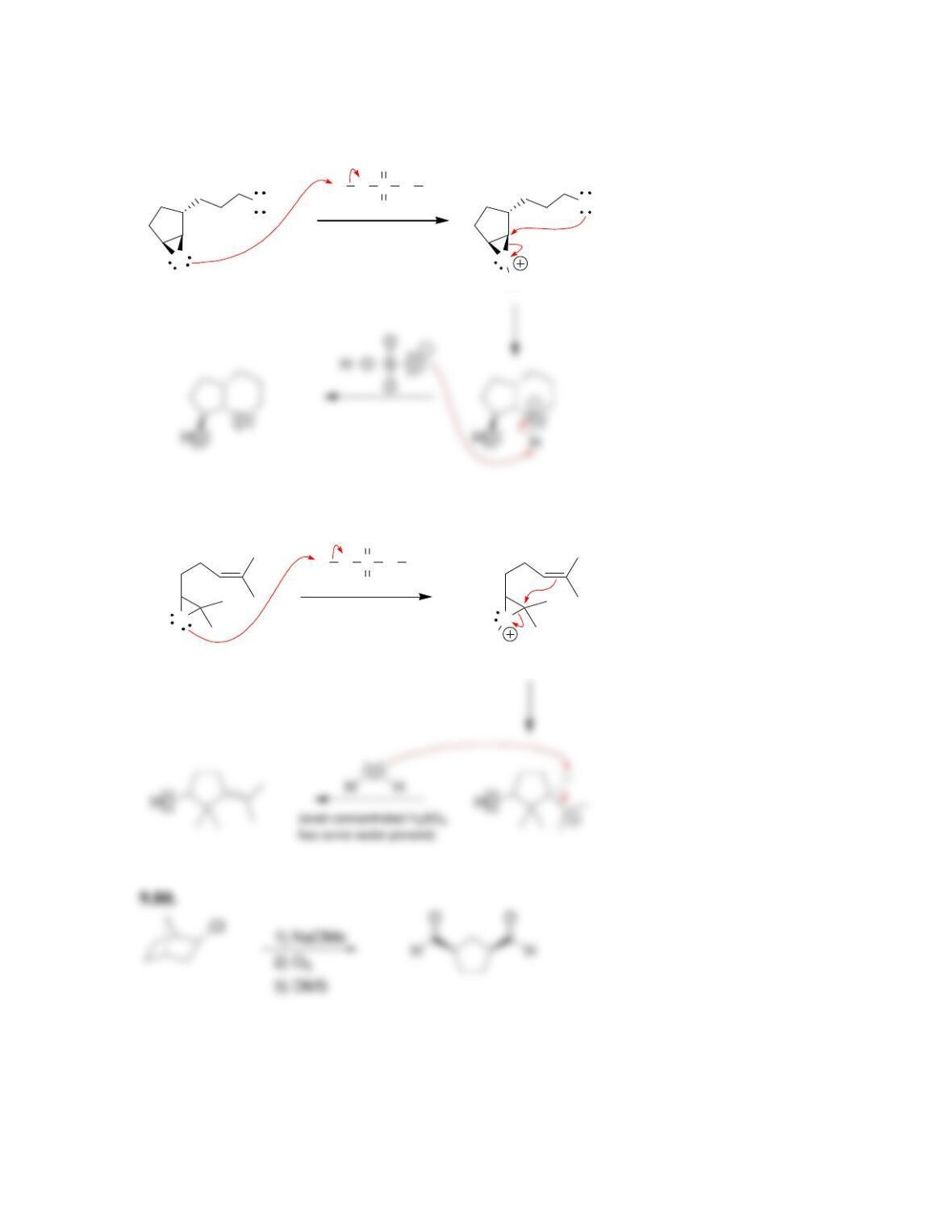
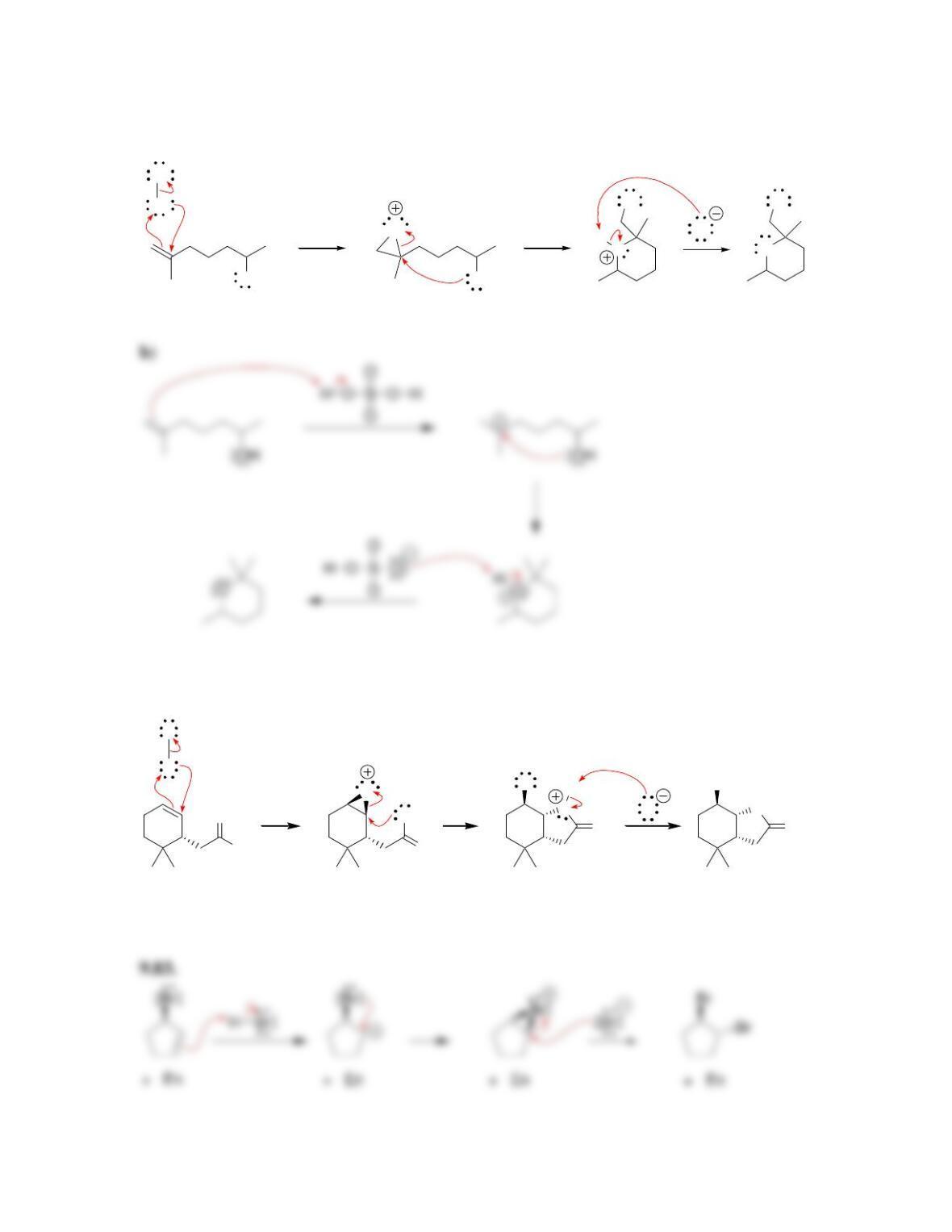
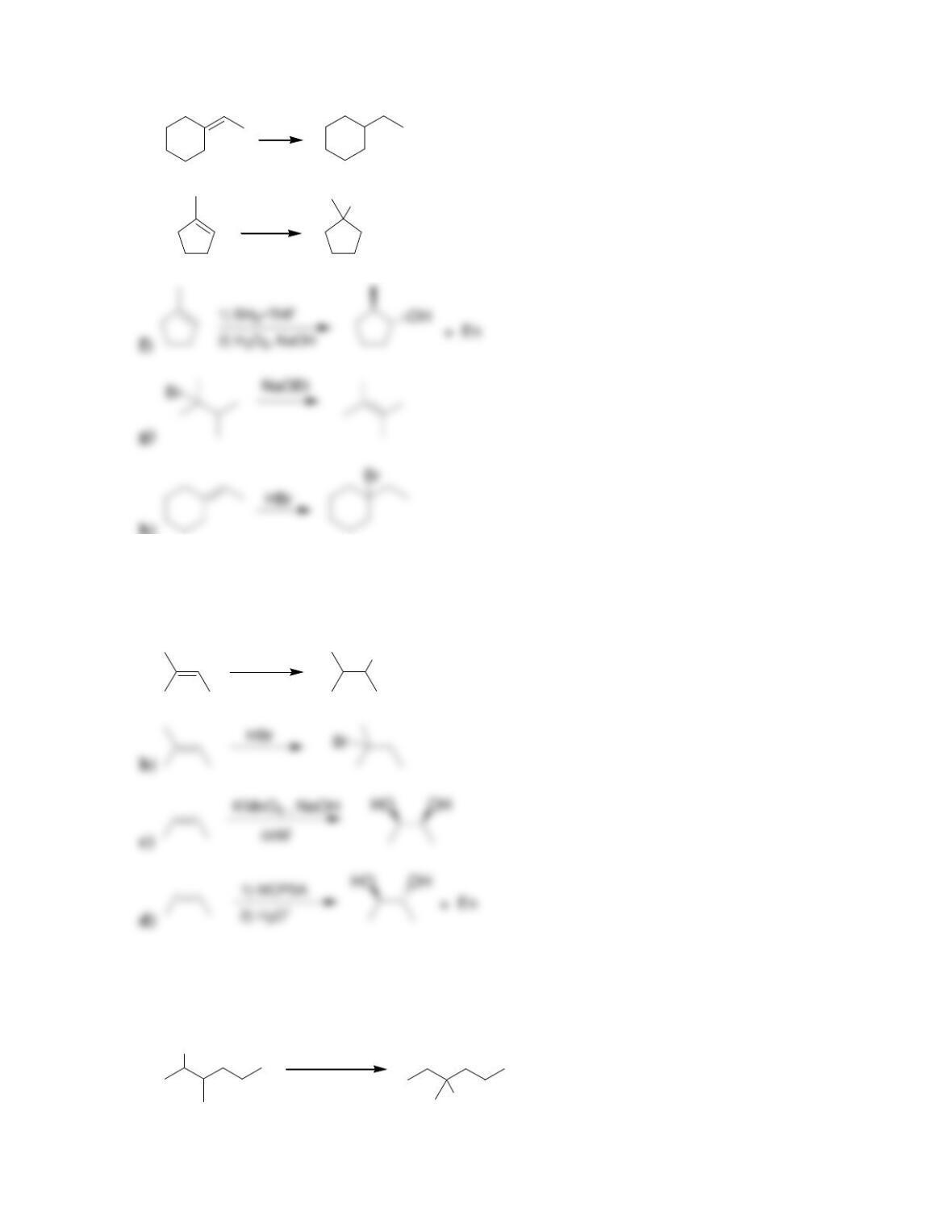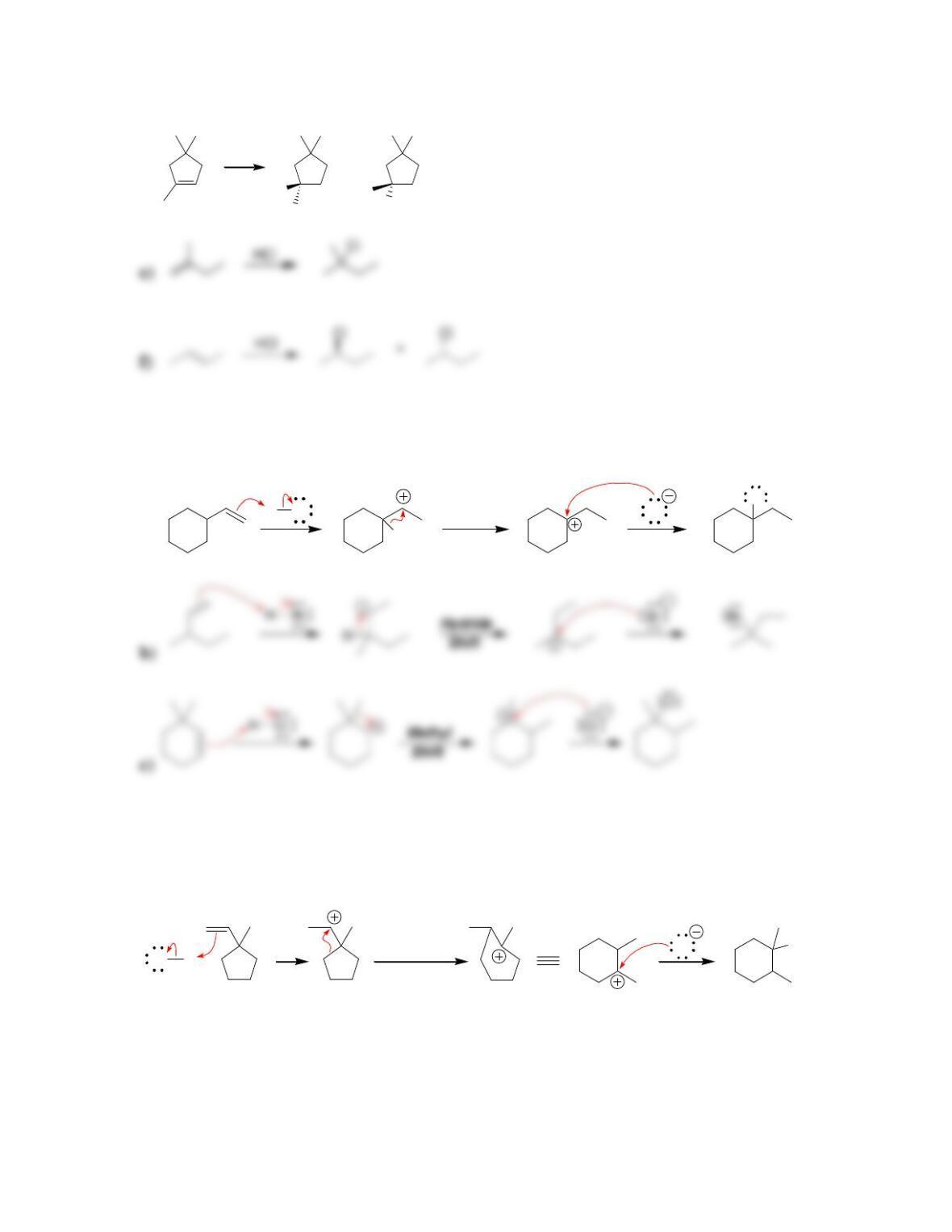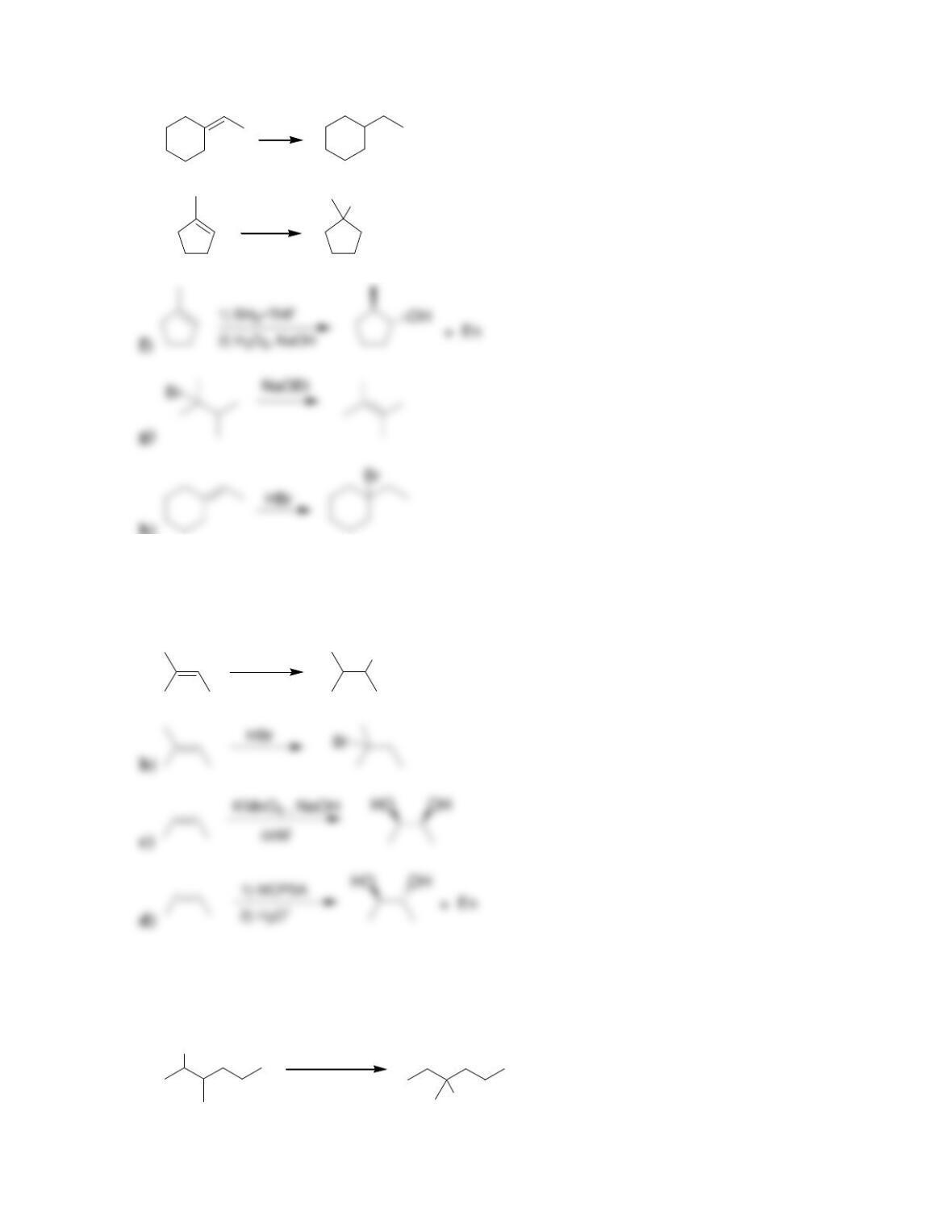Chapter 9
Addition Reactions of Alkenes
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 9. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• Addition reactions are thermodynamically favorable at ____ temperature and
disfavored at _____ temperature.
• Hydrohalogenation reactions are regioselective, because the halogen is generally
placed at the ______ substituted position, called _______________ addition.
• In the presence of _____________, addition of HBr proceeds via an anti-
Markovnikov addition.
• The regioselectivity of an ionic addition reaction is determined by the preference
Review of Skills
Fill in the blanks and empty boxes below. To verify that your answers are correct, look
in your textbook at the end of Chapter 9. The answers appear in the section entitled
SkillBuilder Review.
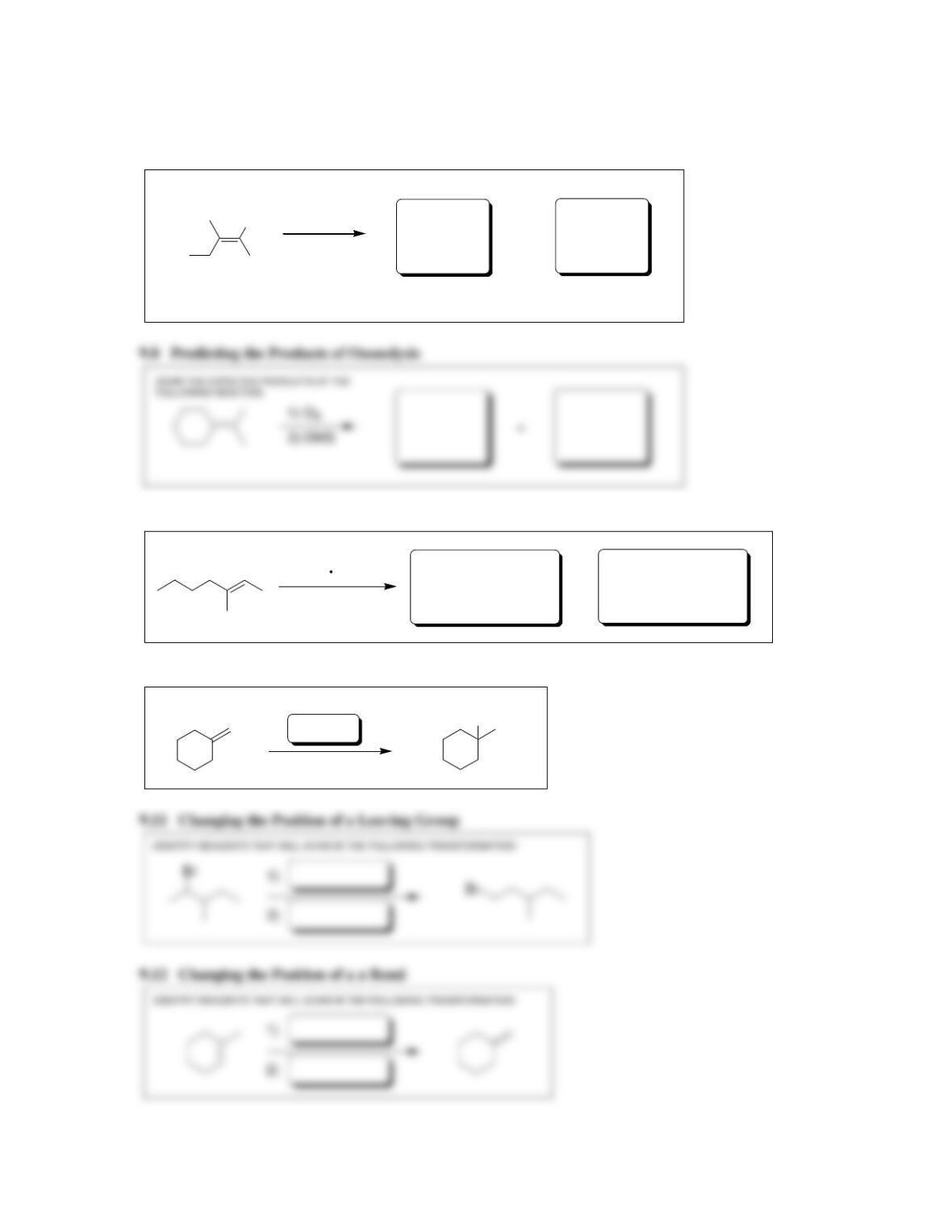
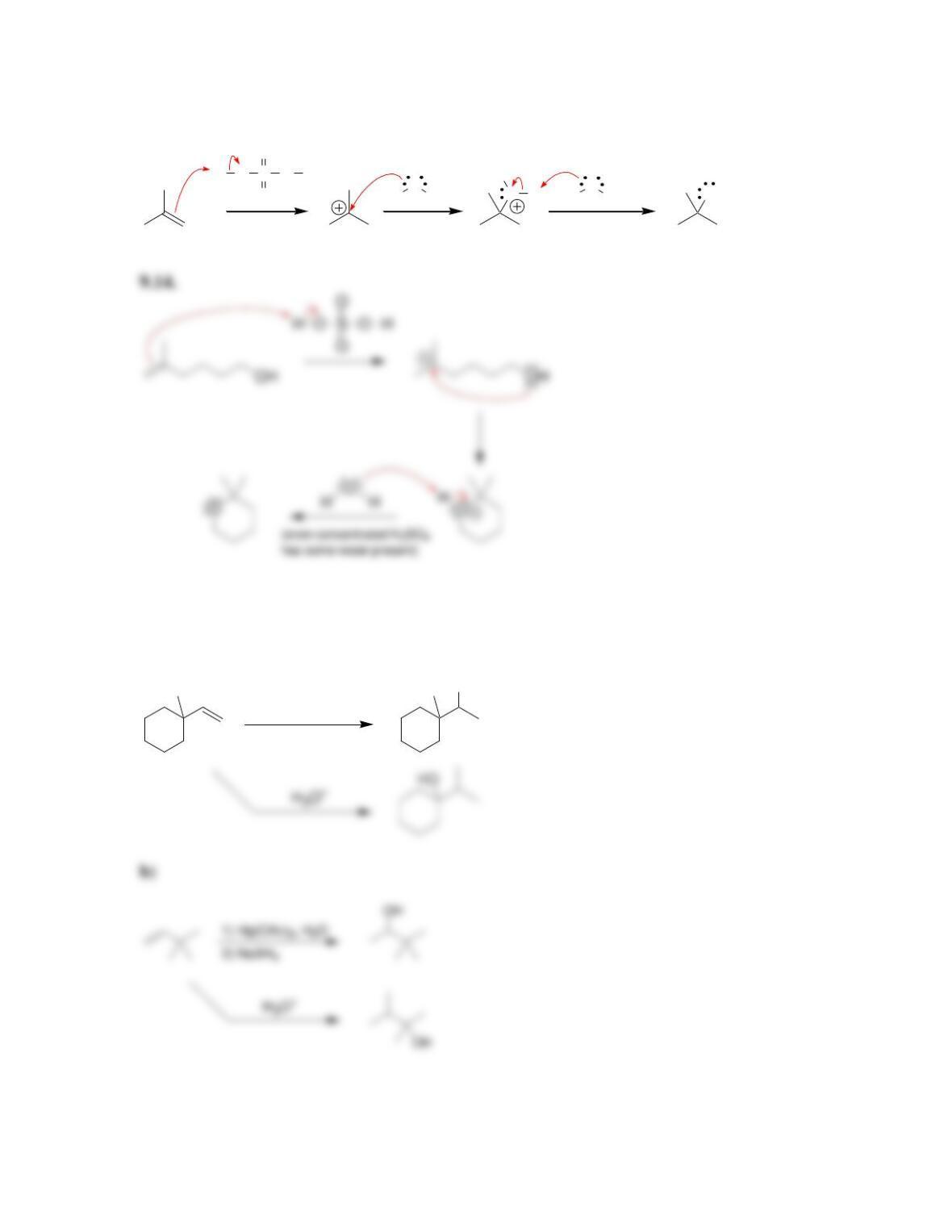
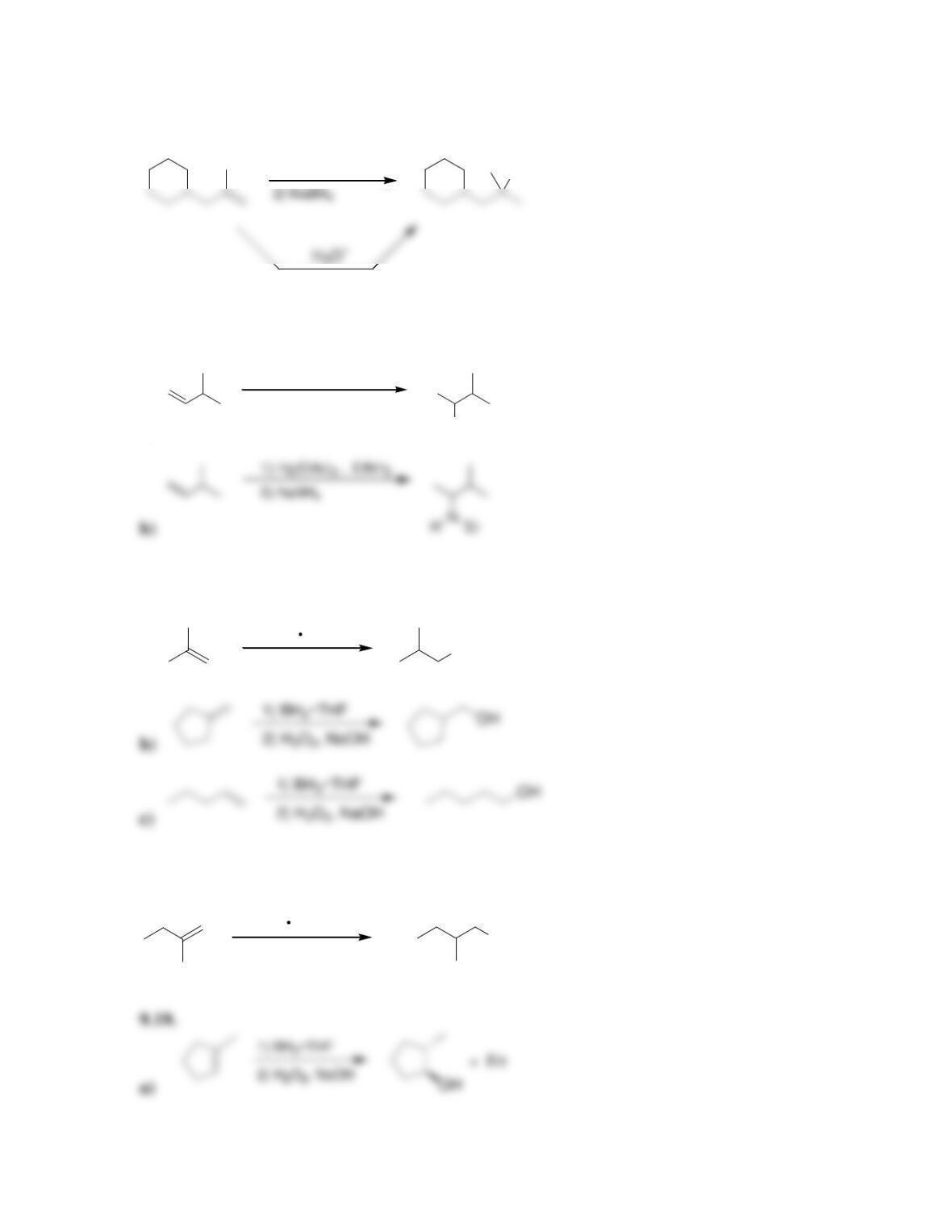
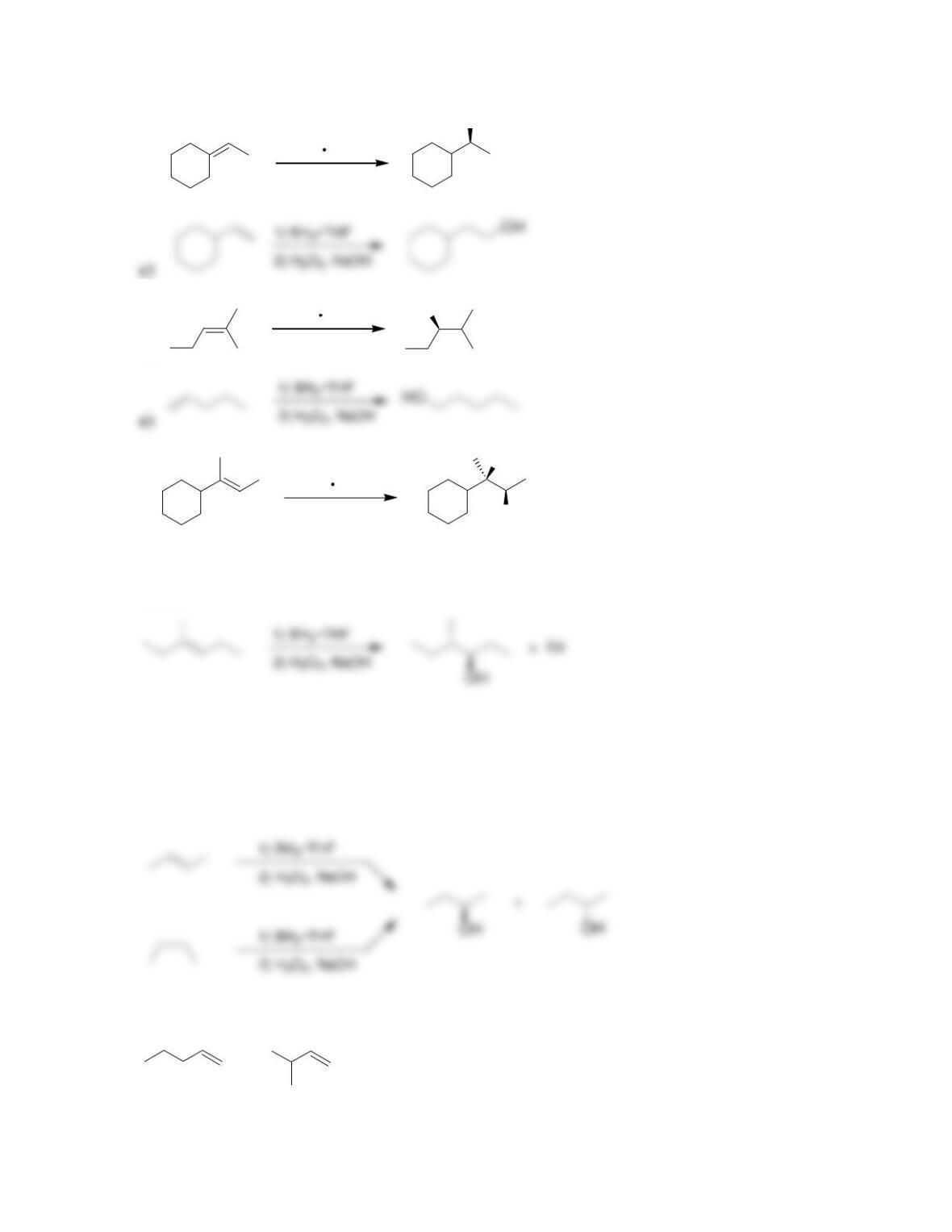
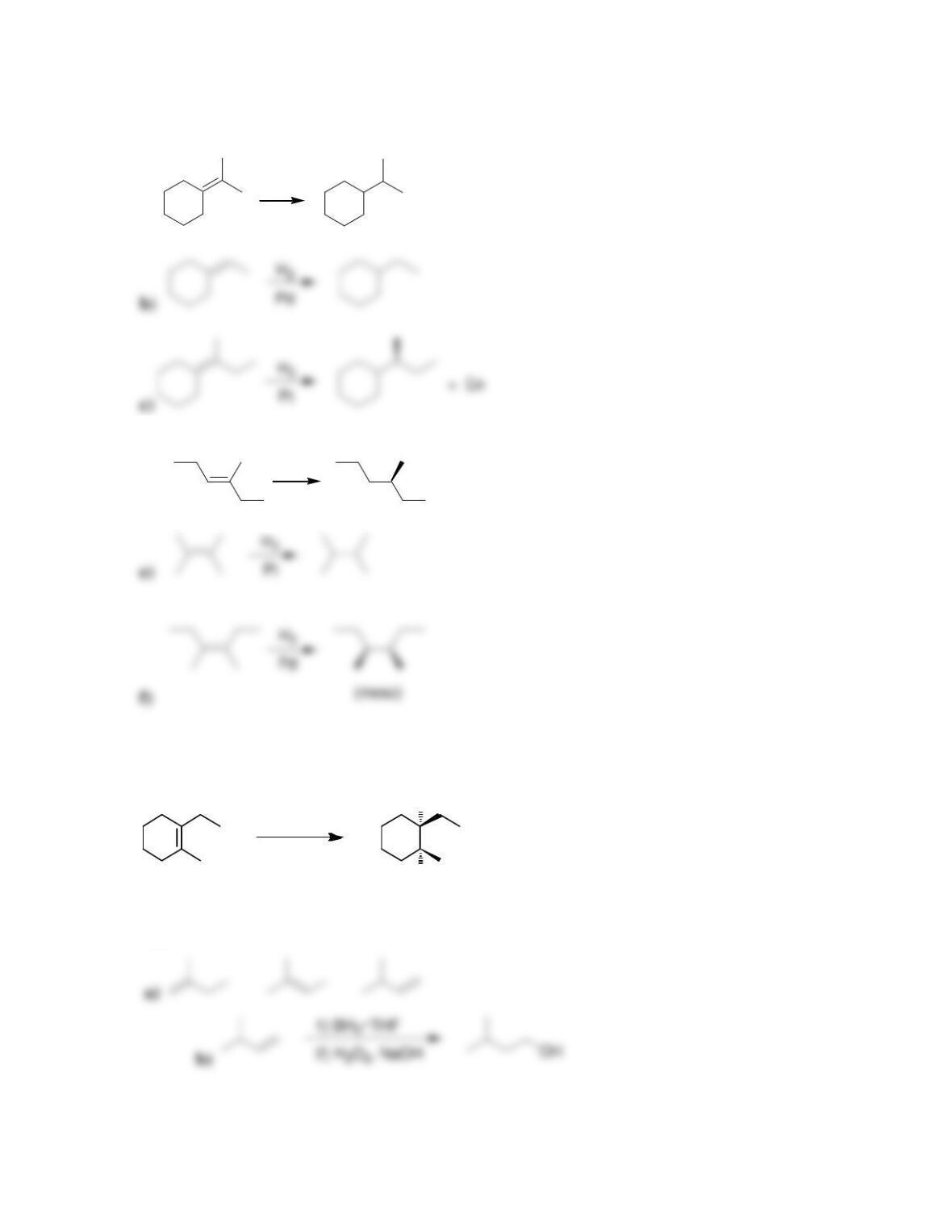
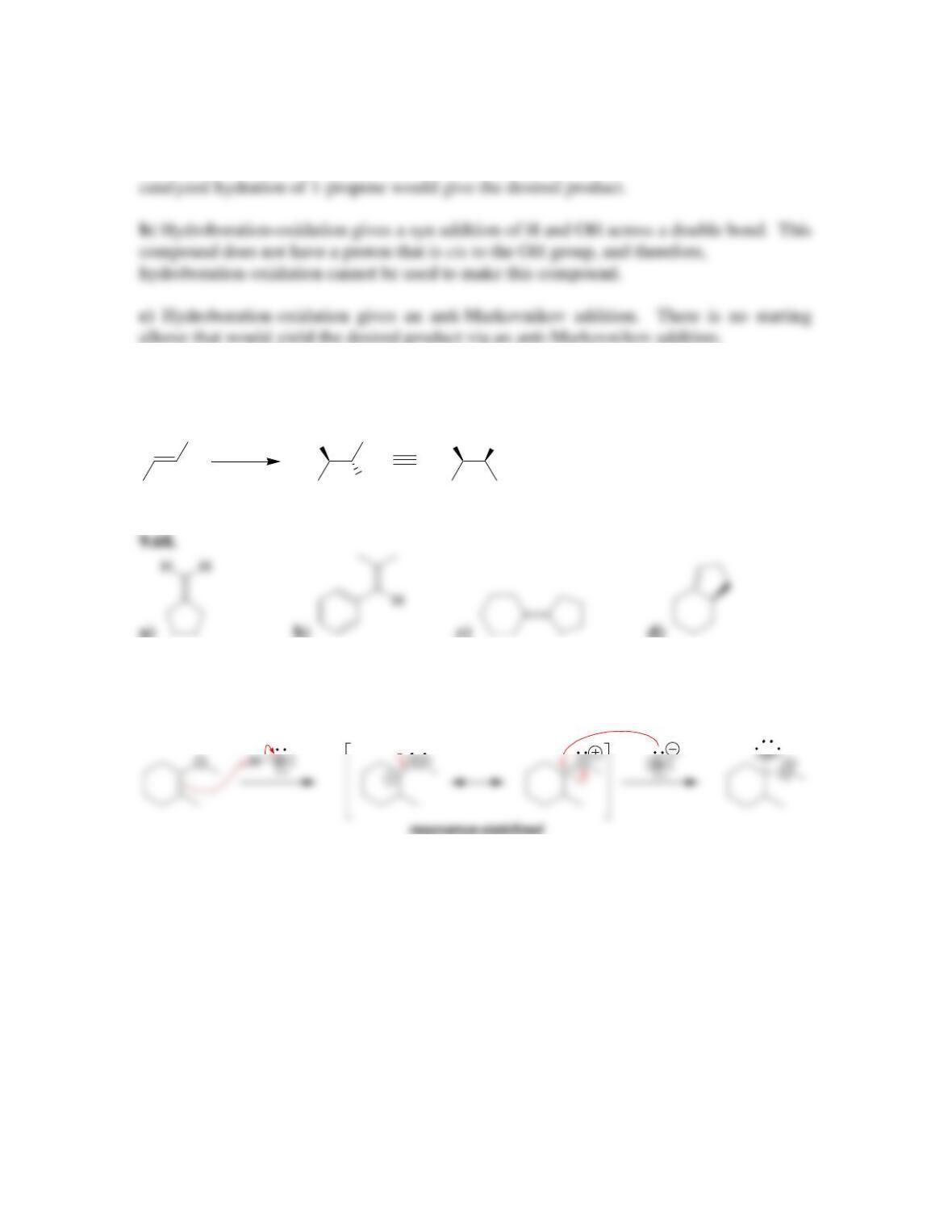
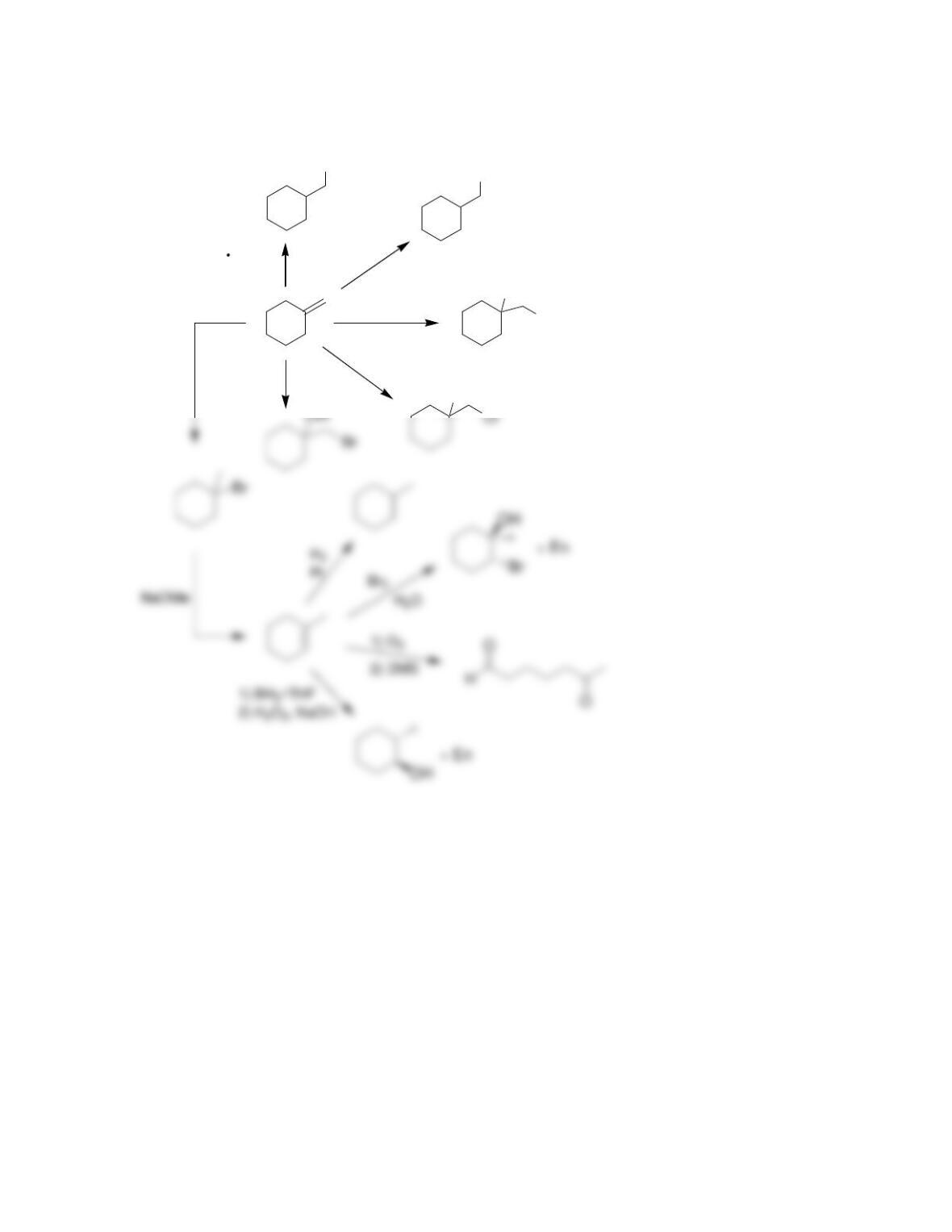
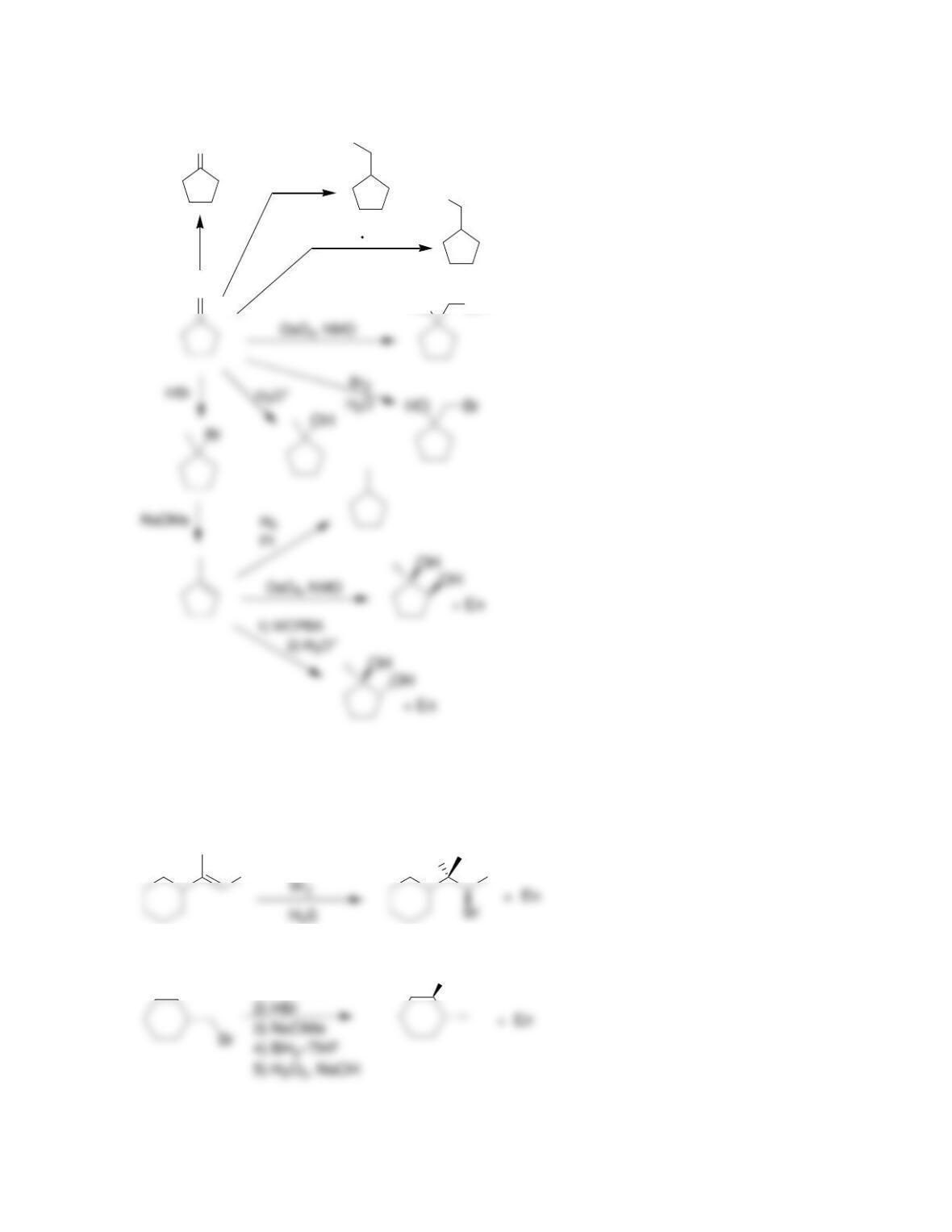
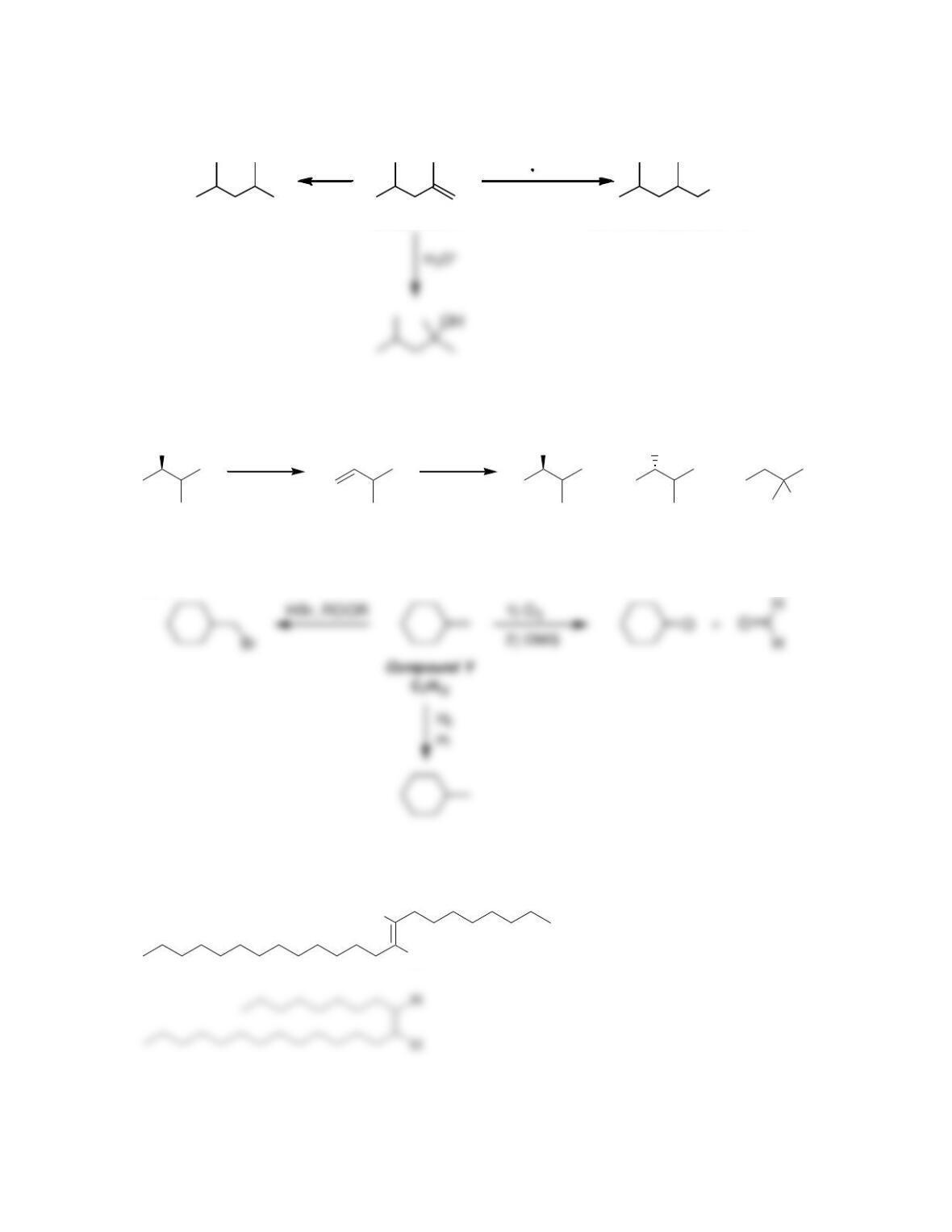
9.1 Drawing a Mechanism for Hydrohalogenation