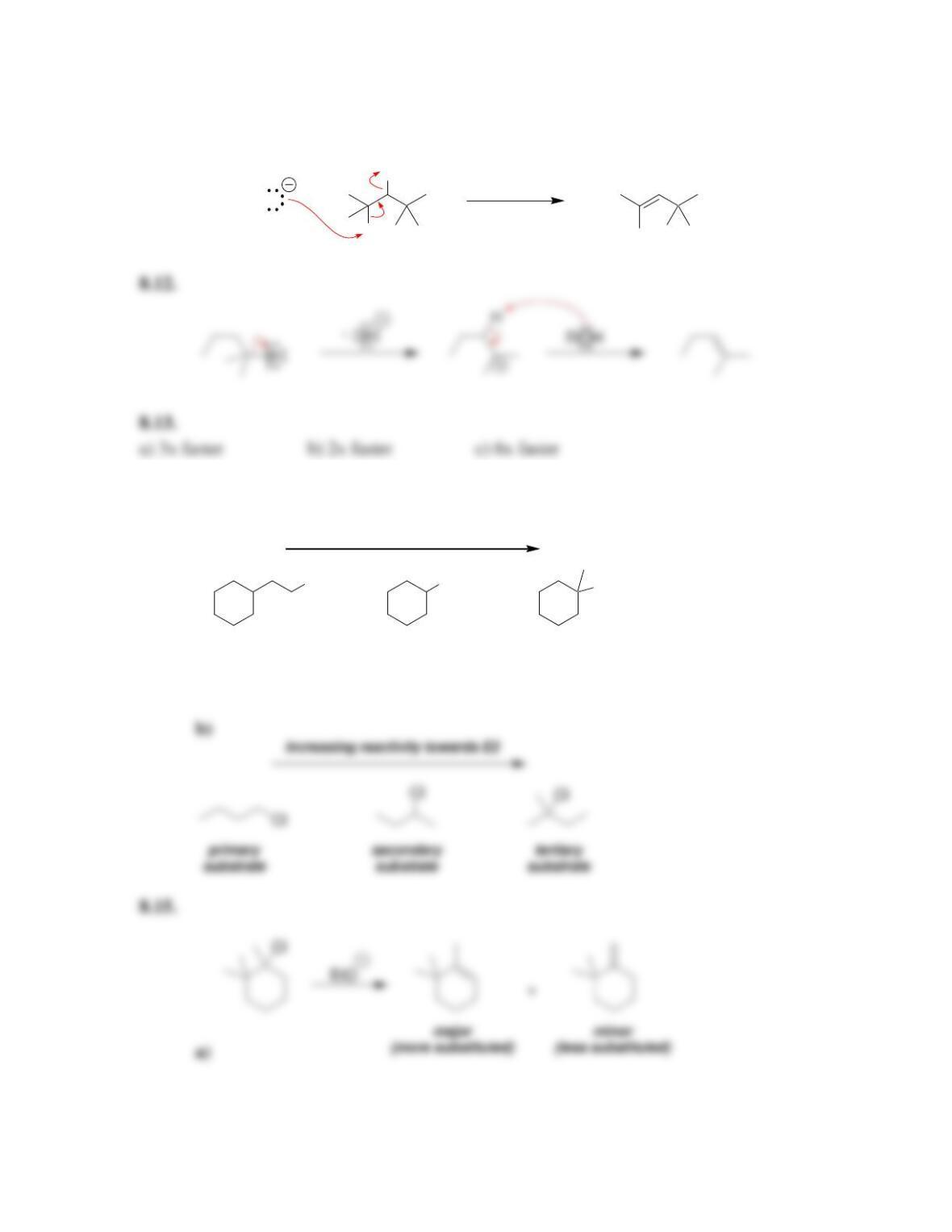
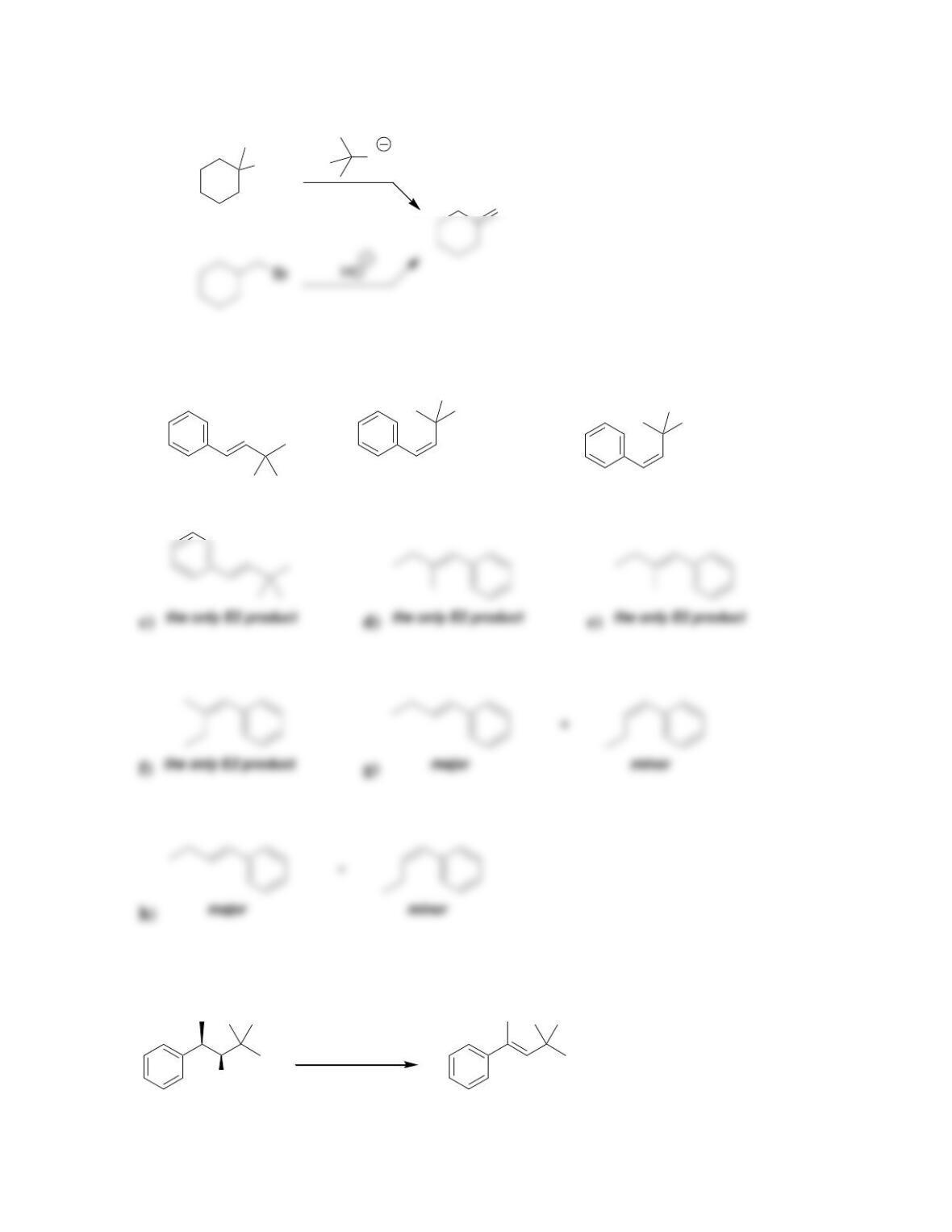
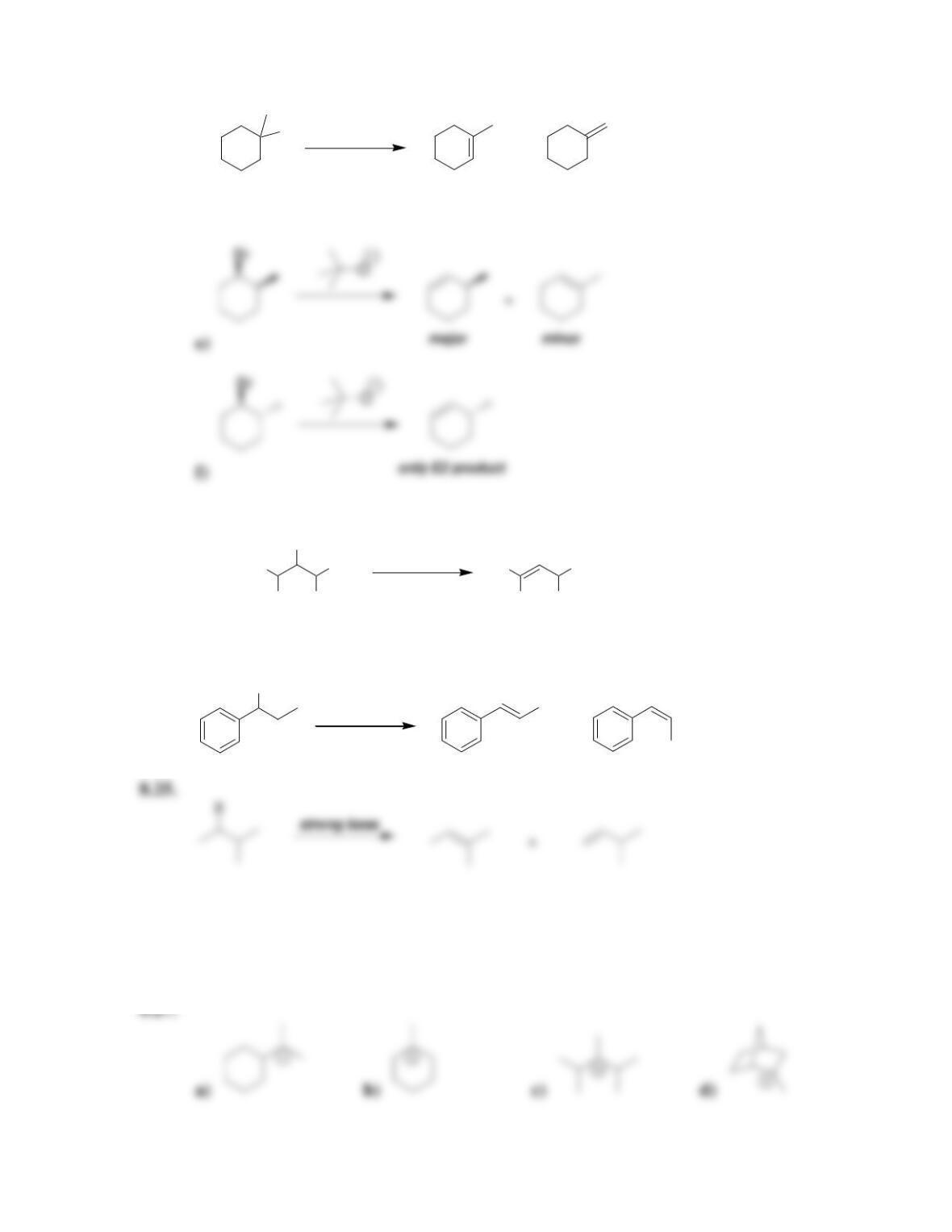
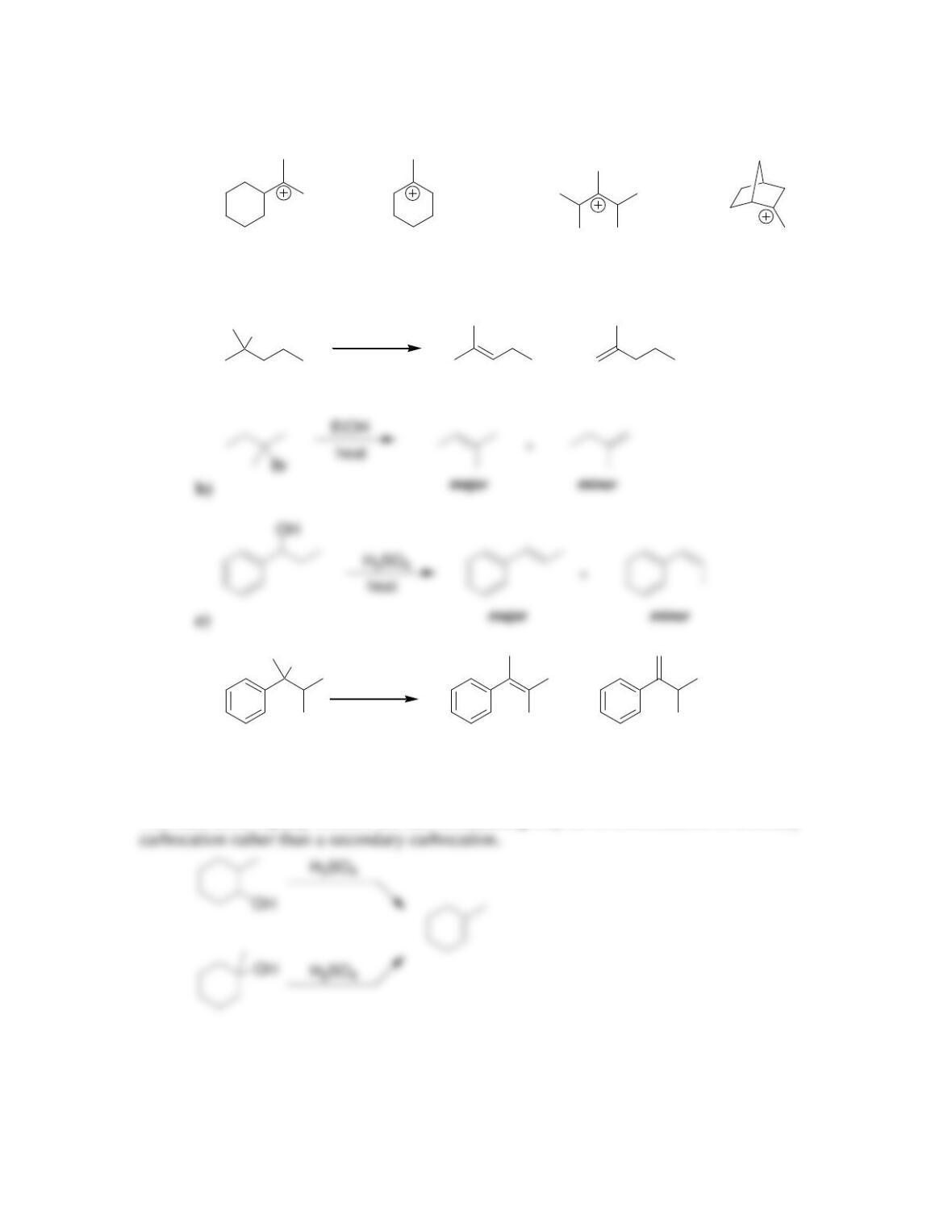
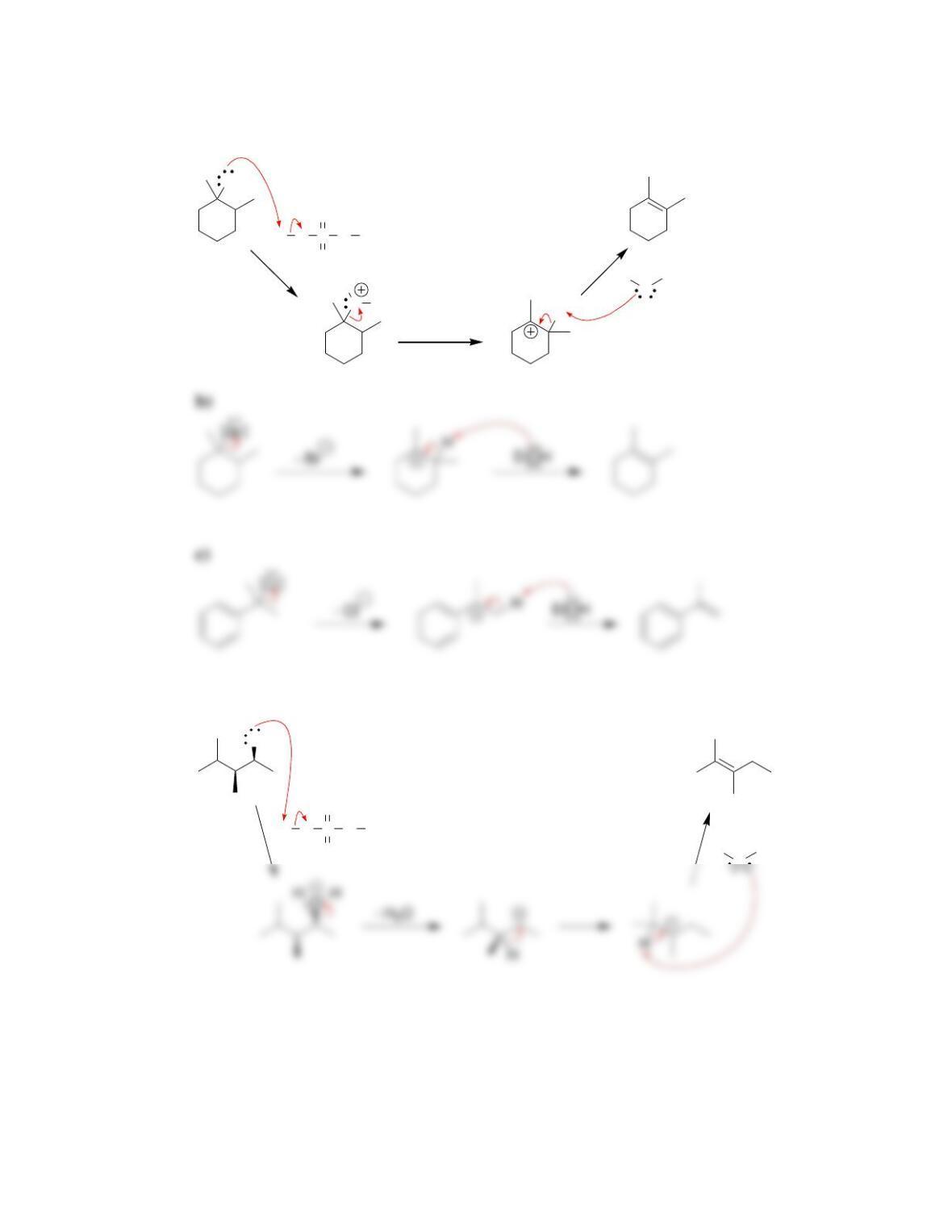
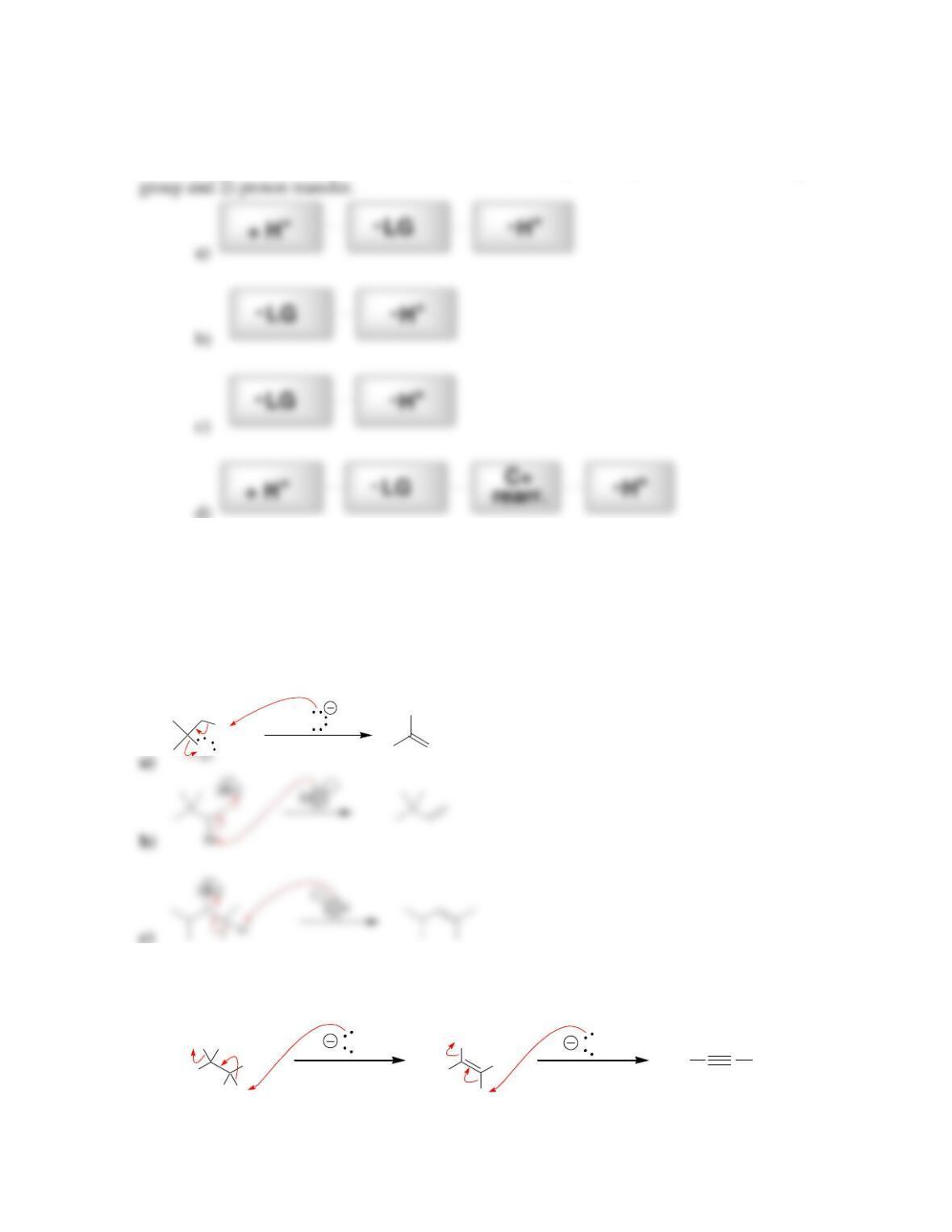
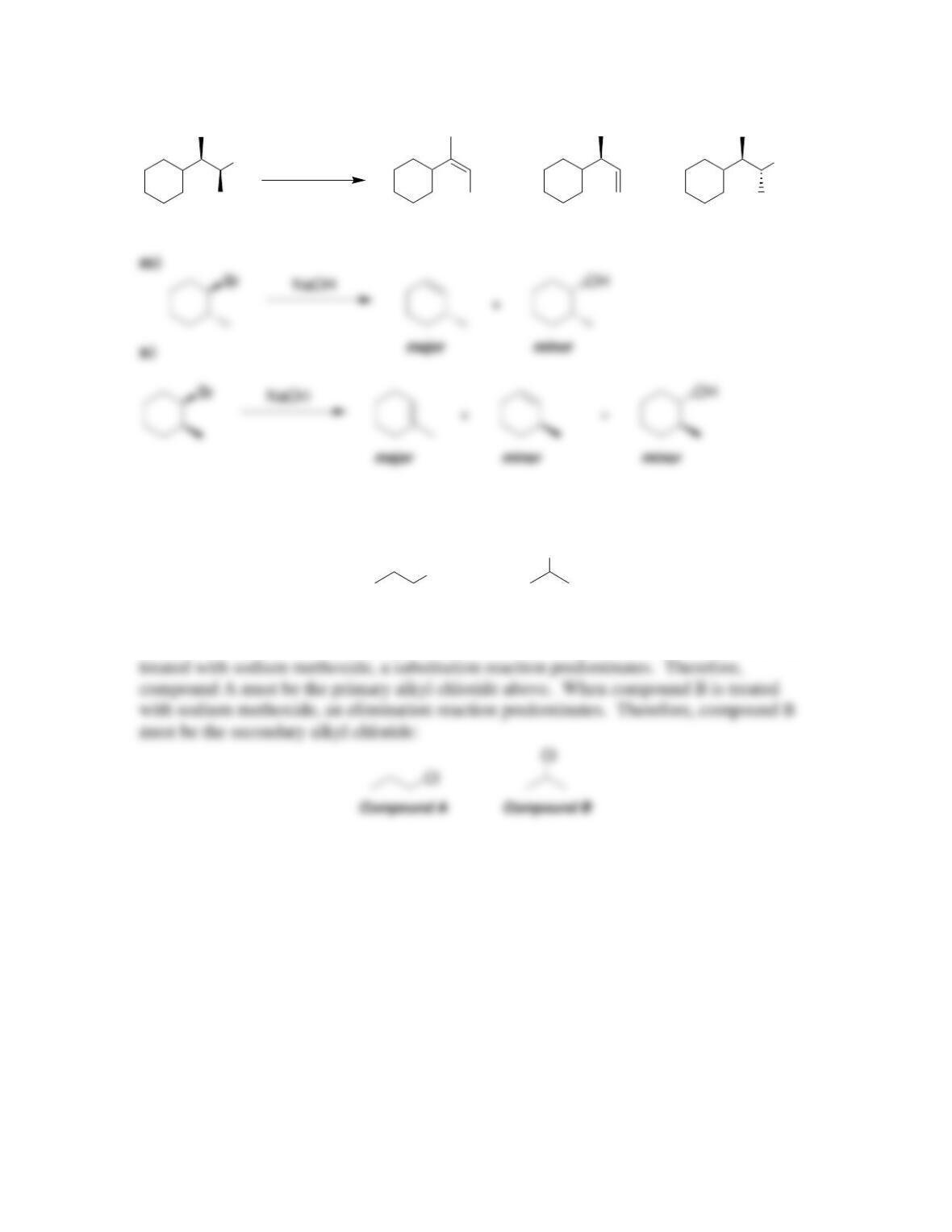
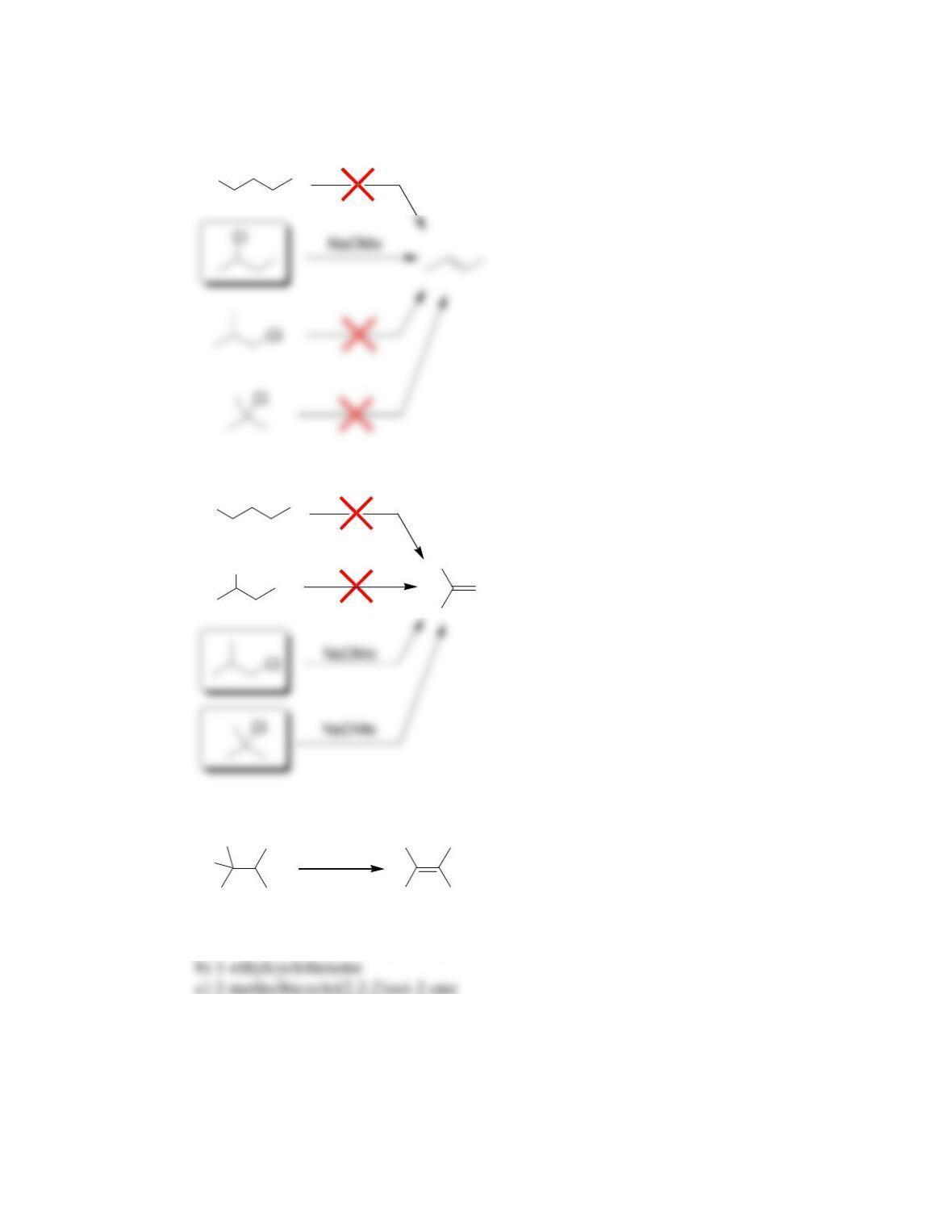
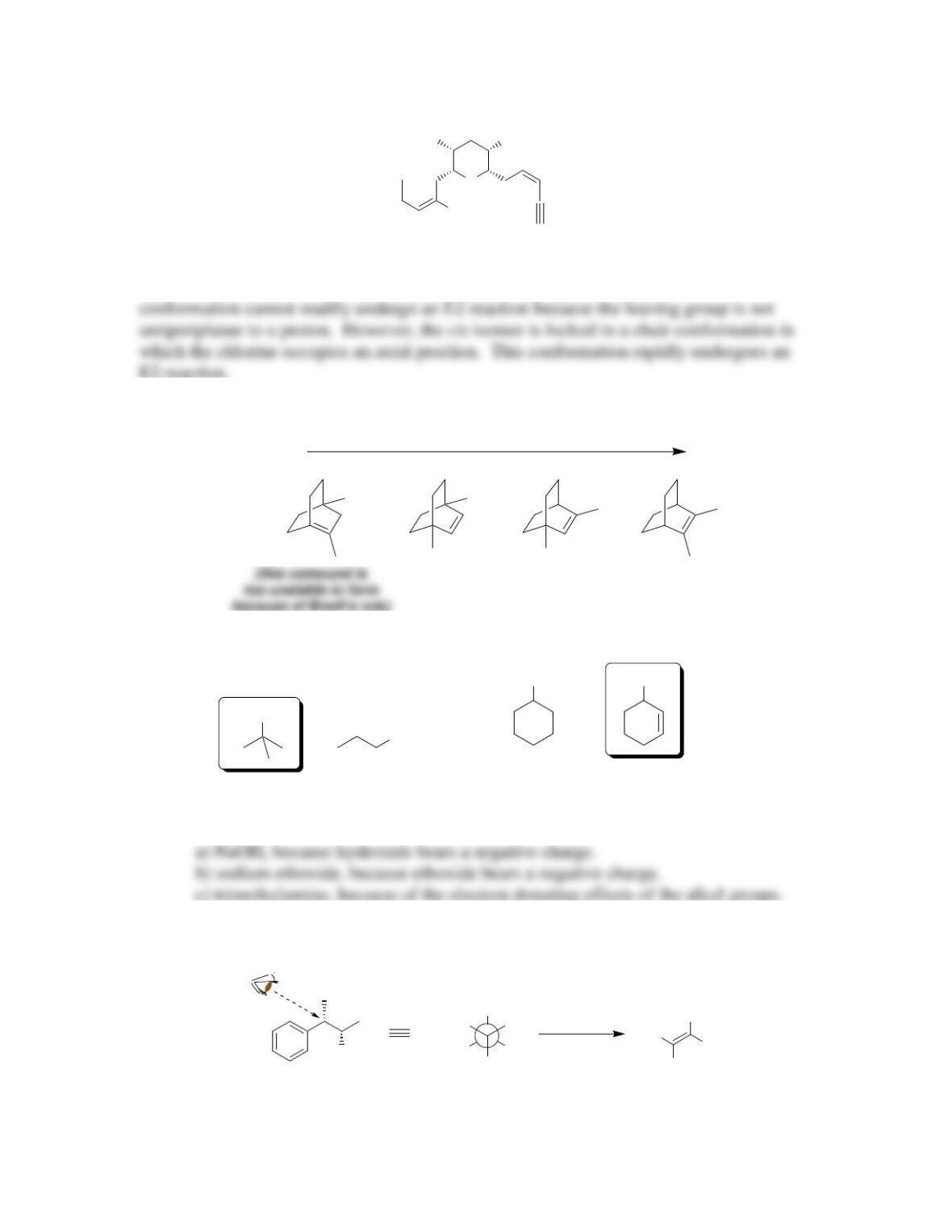
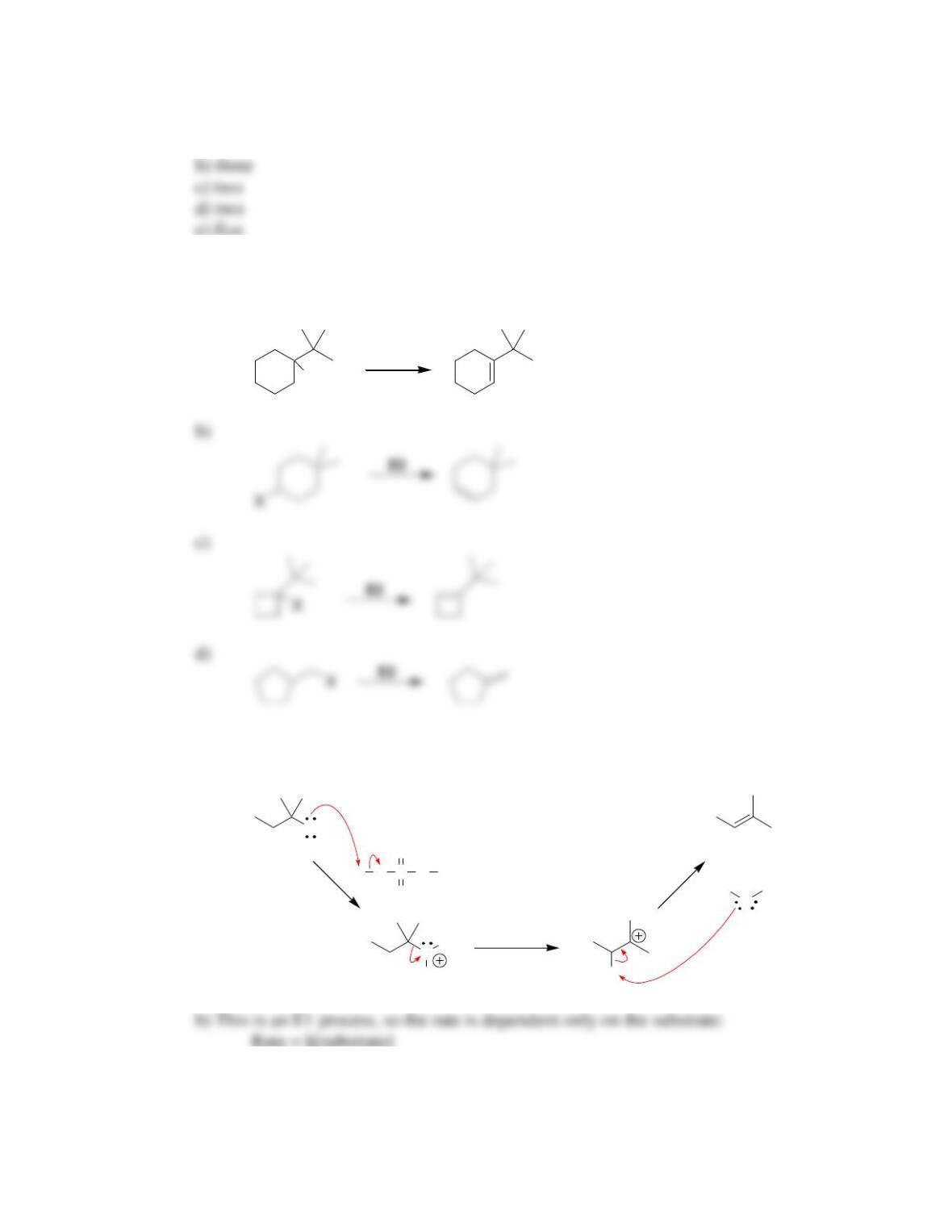
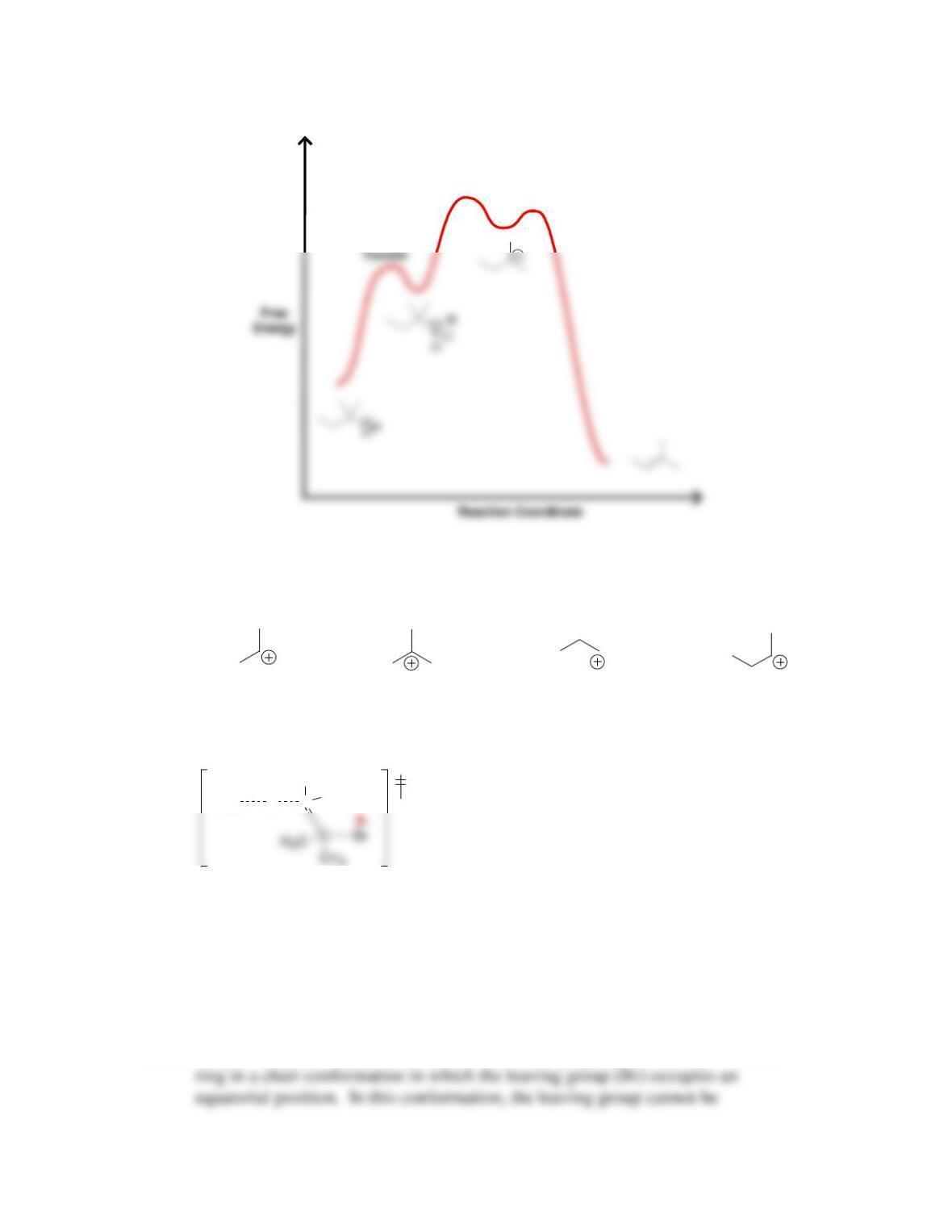
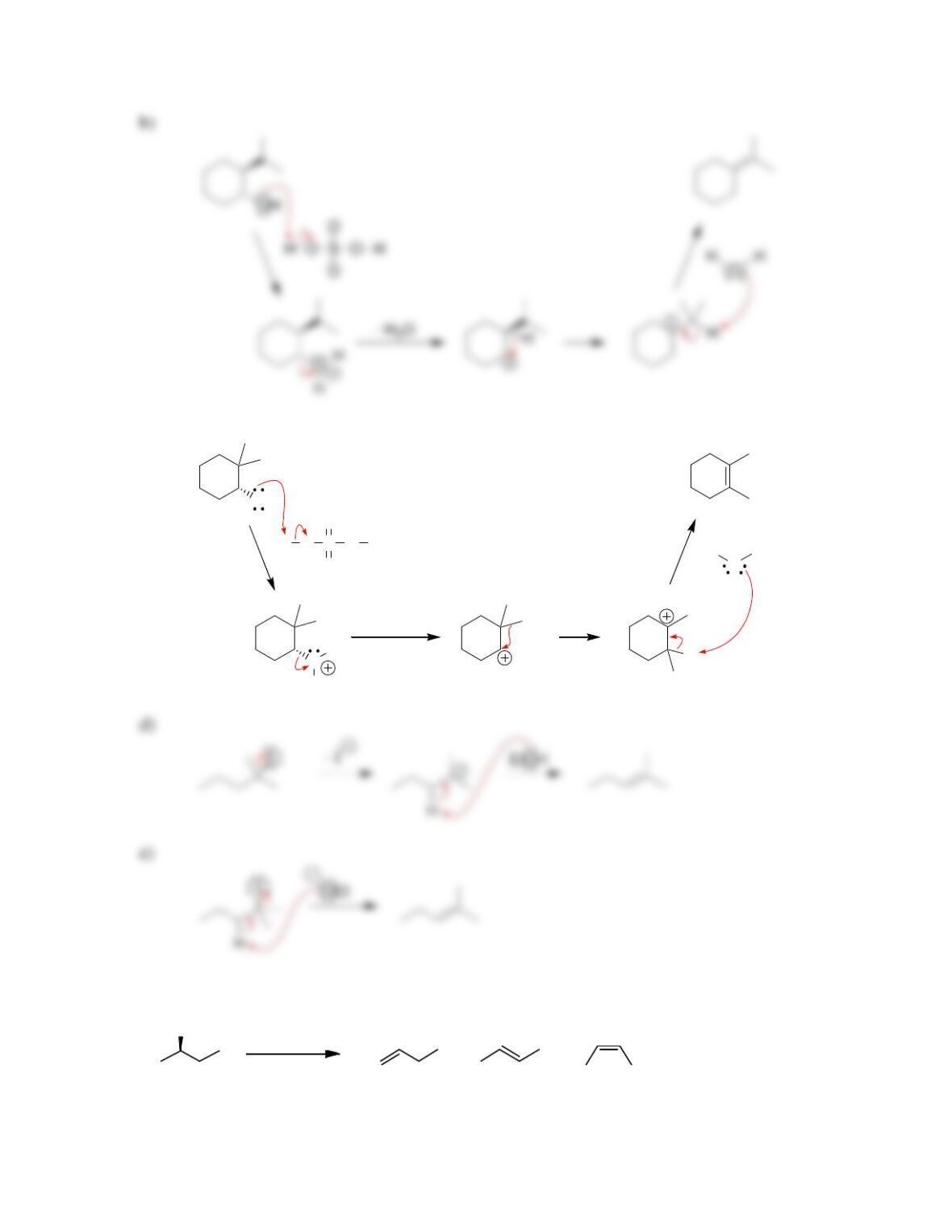
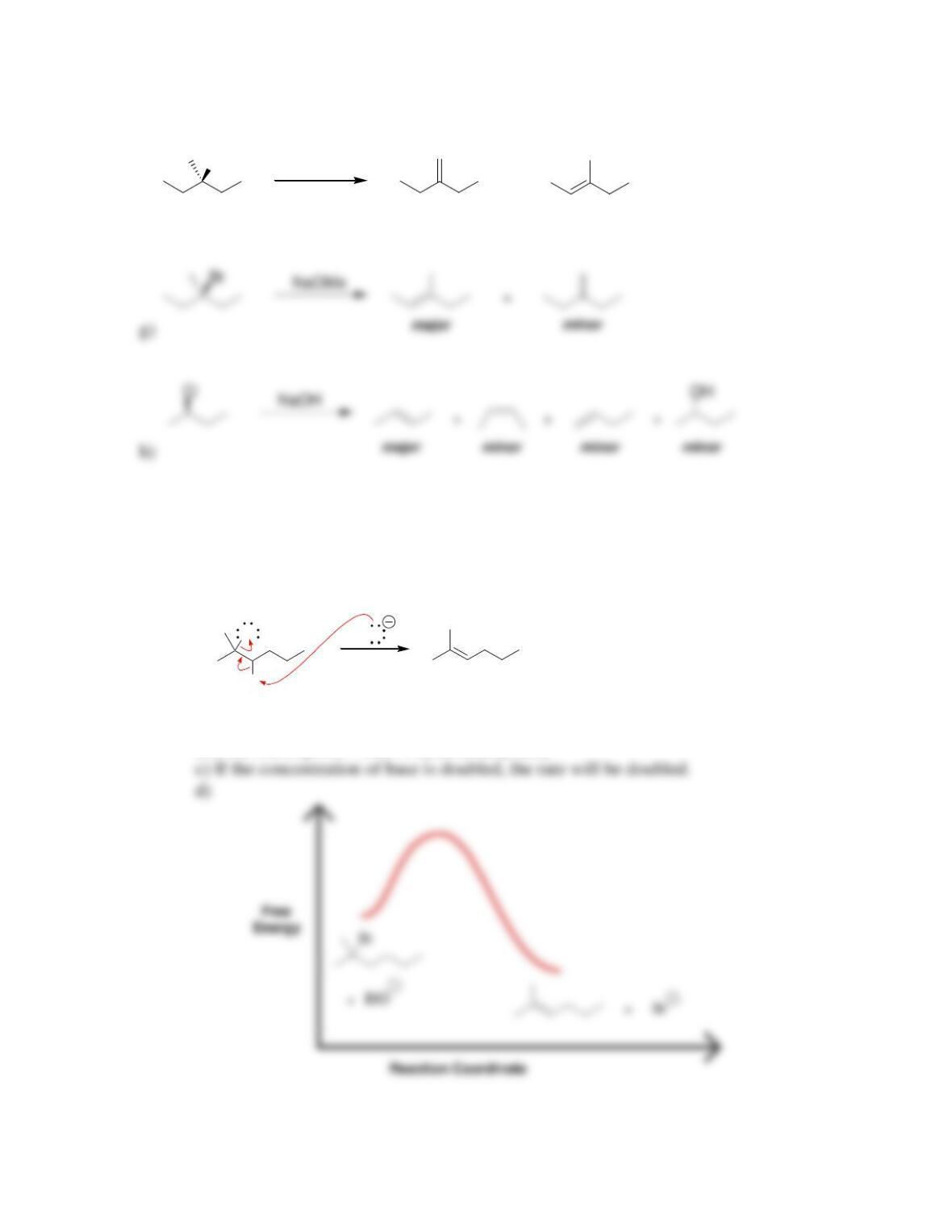
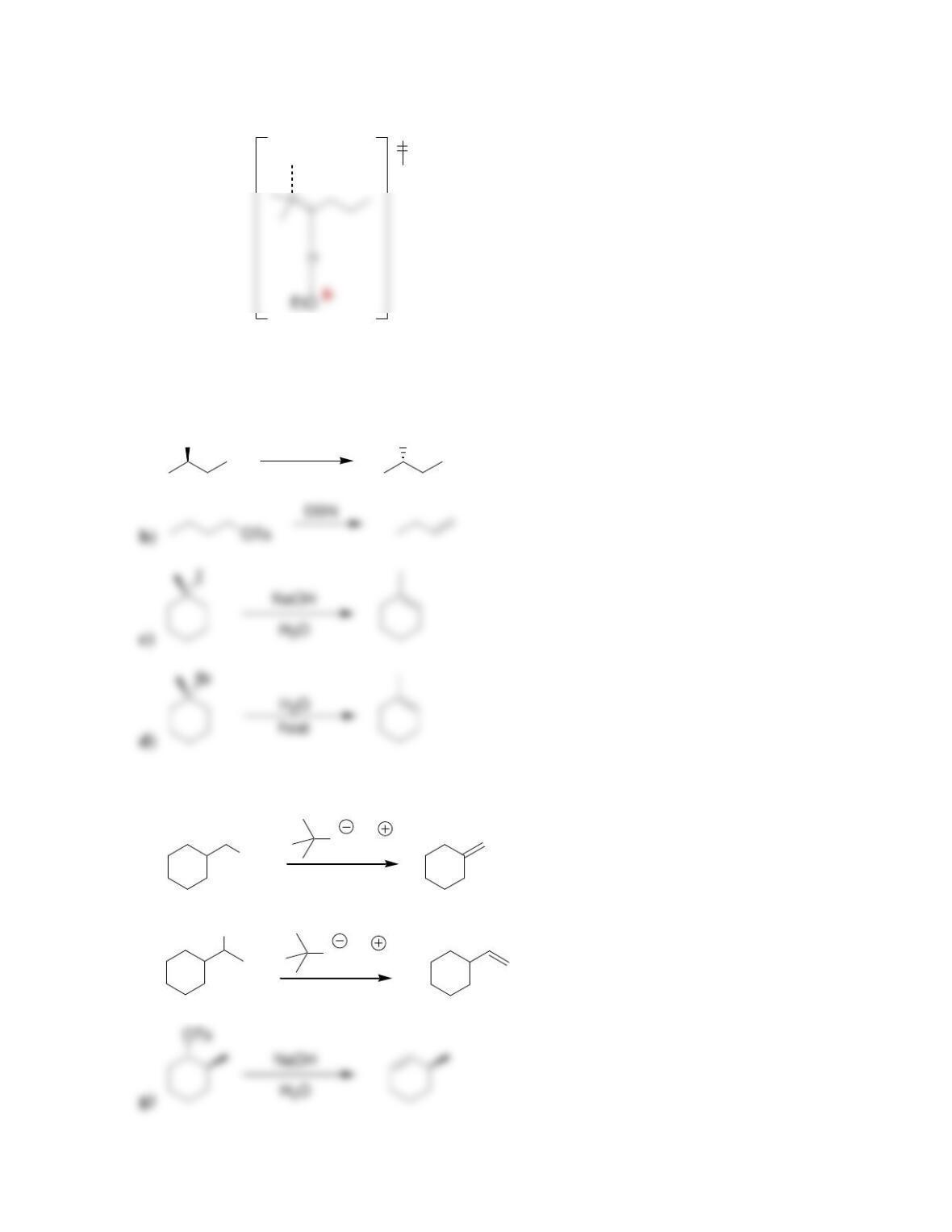
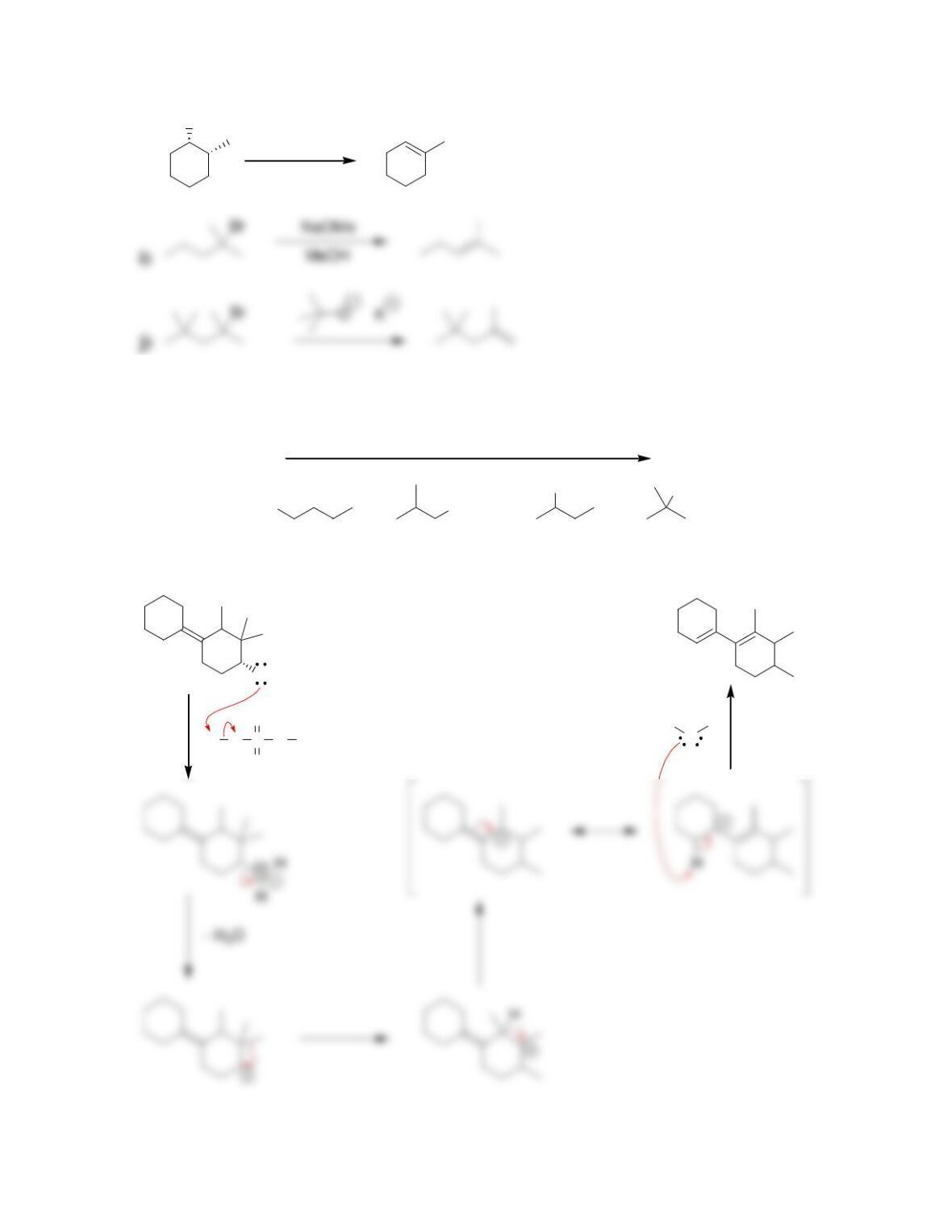
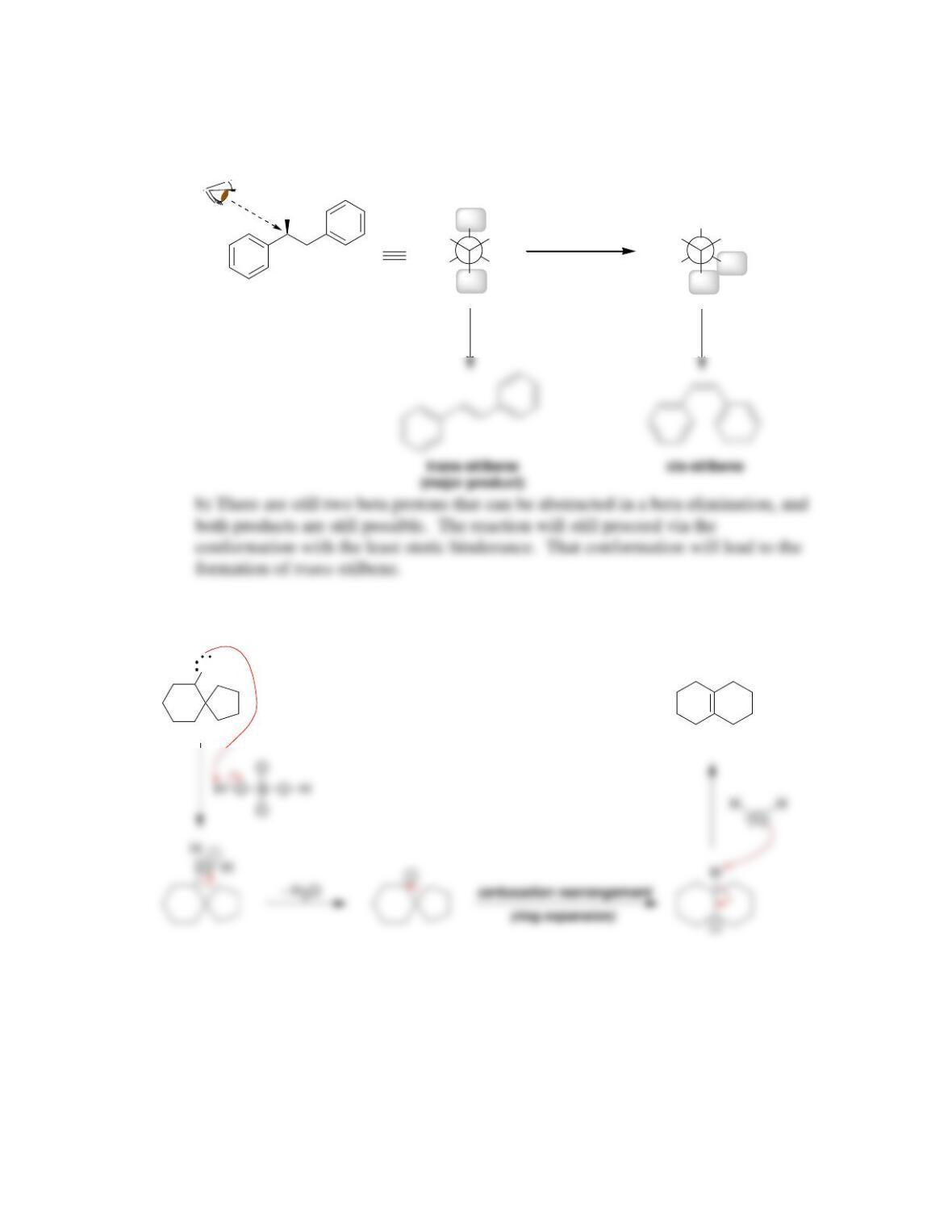
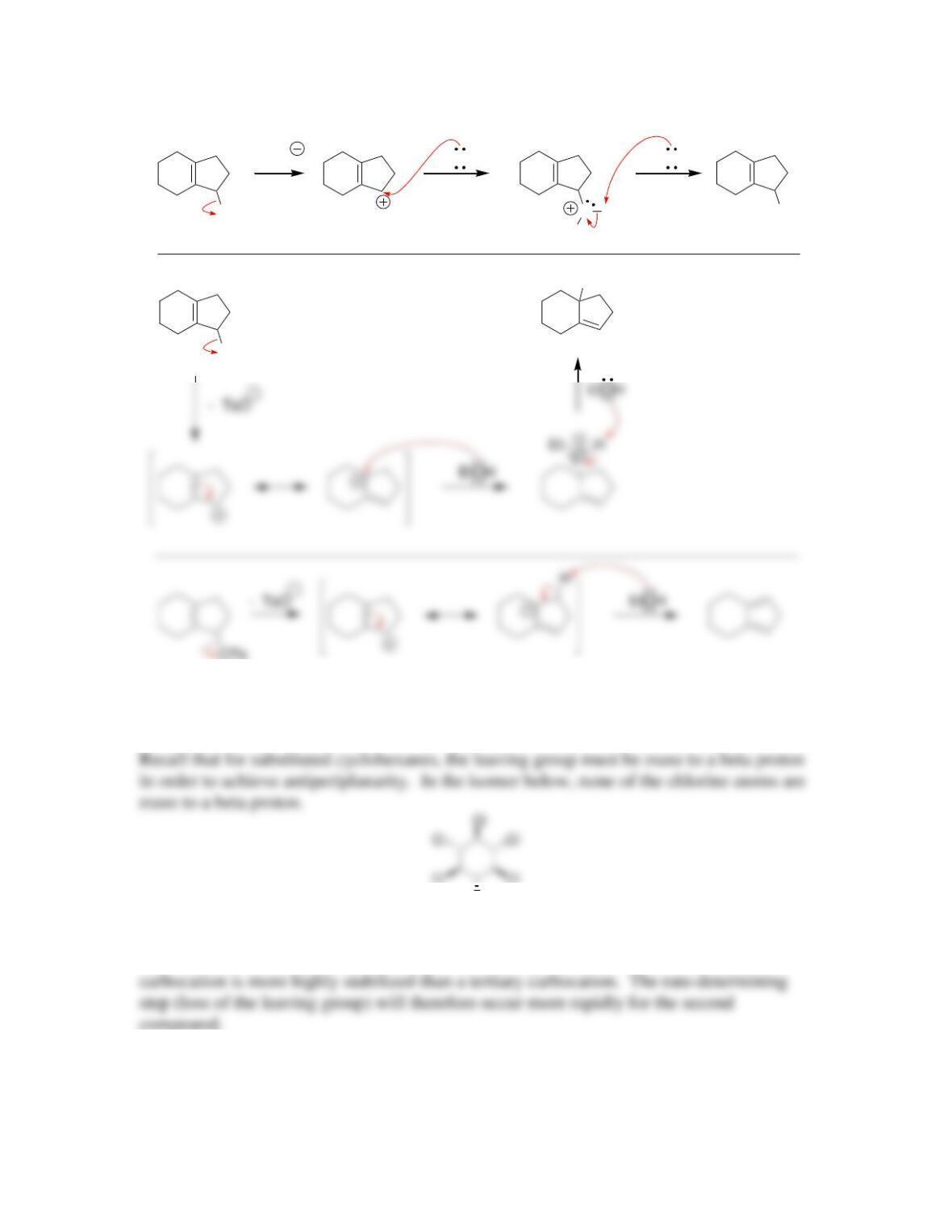
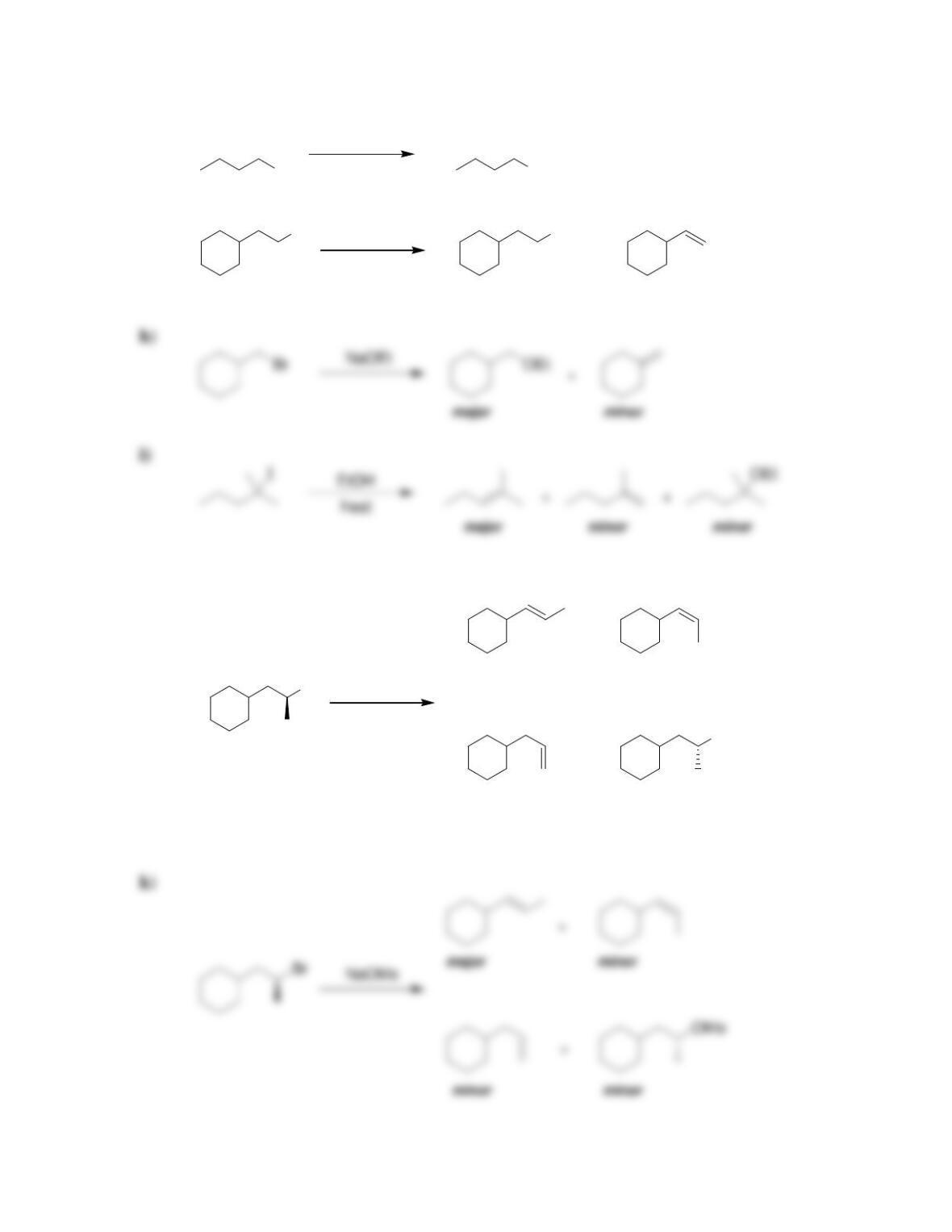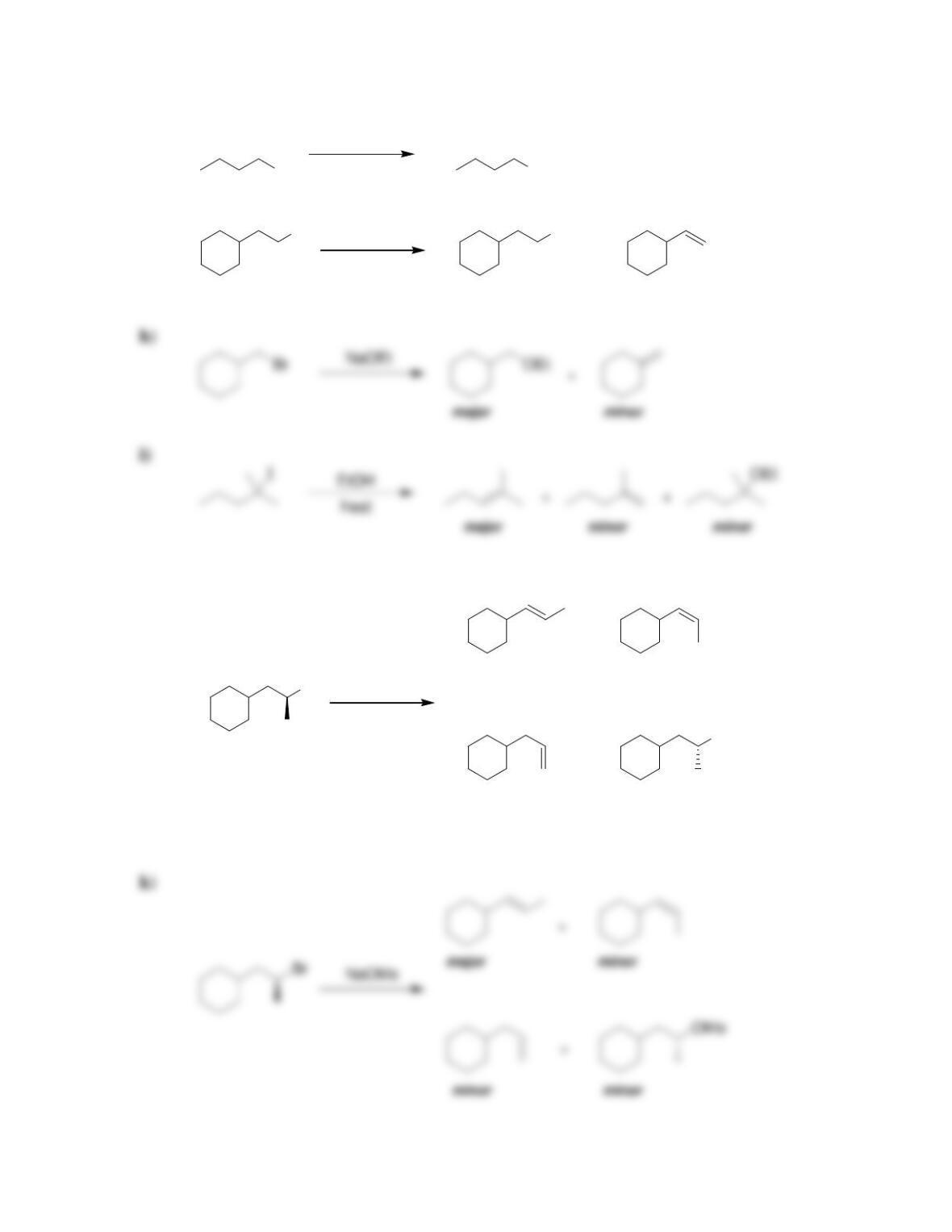Chapter 8
Alkenes: Structure and
Preparation via Elimination Reactions
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 8. Each of the sentences below appears in the section entitled Review
of Concepts and Vocabulary.
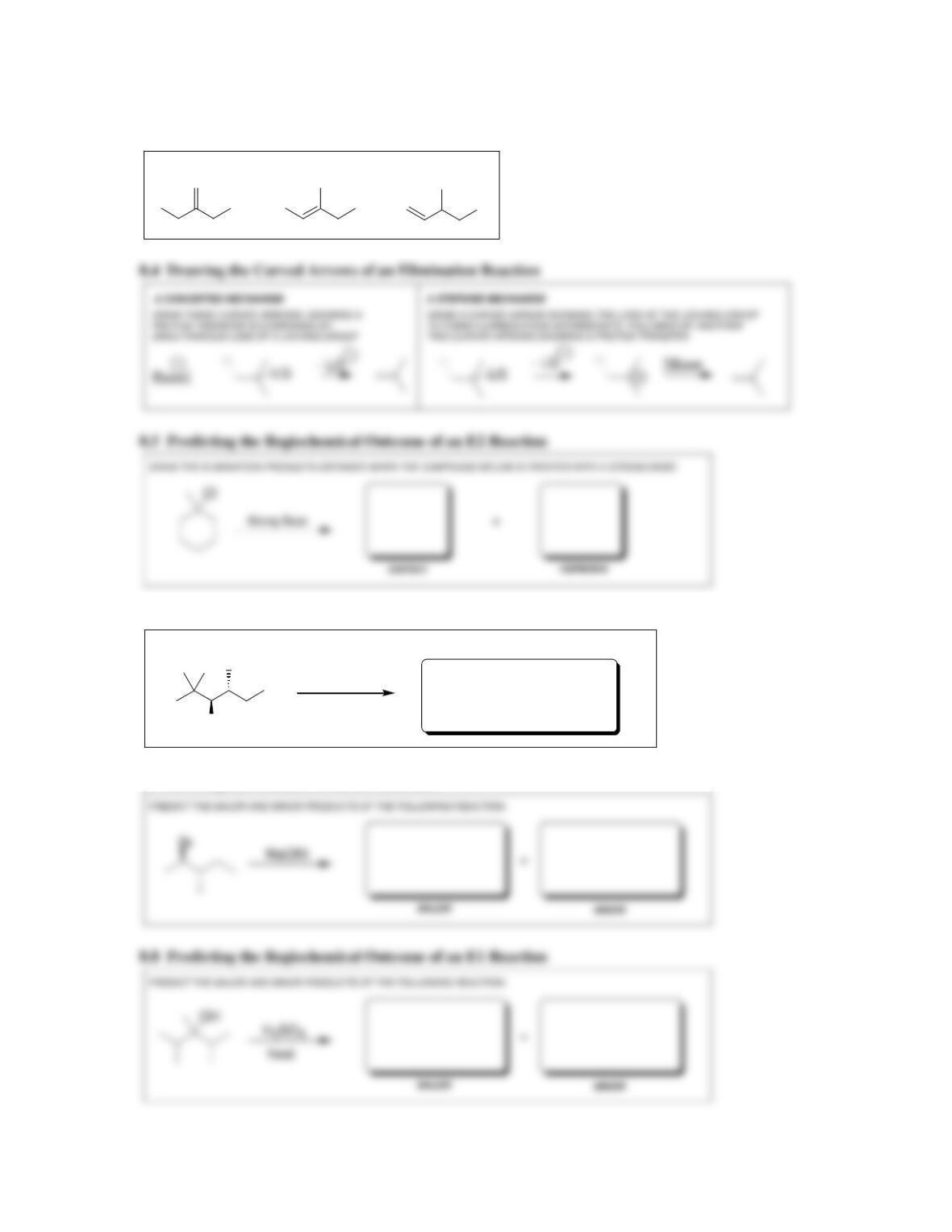
• Alkene stability increases with increasing degree of ________________.
• E2 reactions are said to be regioselective, because the more substituted alkene,
Review of Skills
Fill in the blanks and empty boxes below. To verify that your answers are correct, look
in your textbook at the end of Chapter 8. The answers appear in the section entitled
SkillBuilder Review.
8.1 Assembling the Systematic Name of an Alkene
PROVIDE A SYSTEMATIC NAME FOR THE FOLLOWING COMPOUND
1) IDENTIFY THE PARENT
2) IDENTIFY AND NAME SUBSTITUENTS
3) ASSIGN LOCANTS TO EACH SUBSTITUENT
4) ALPHABETIZE