Chapter 7
Substitution Reactions
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 7. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• Substitution reactions exchange one _____________ for another.
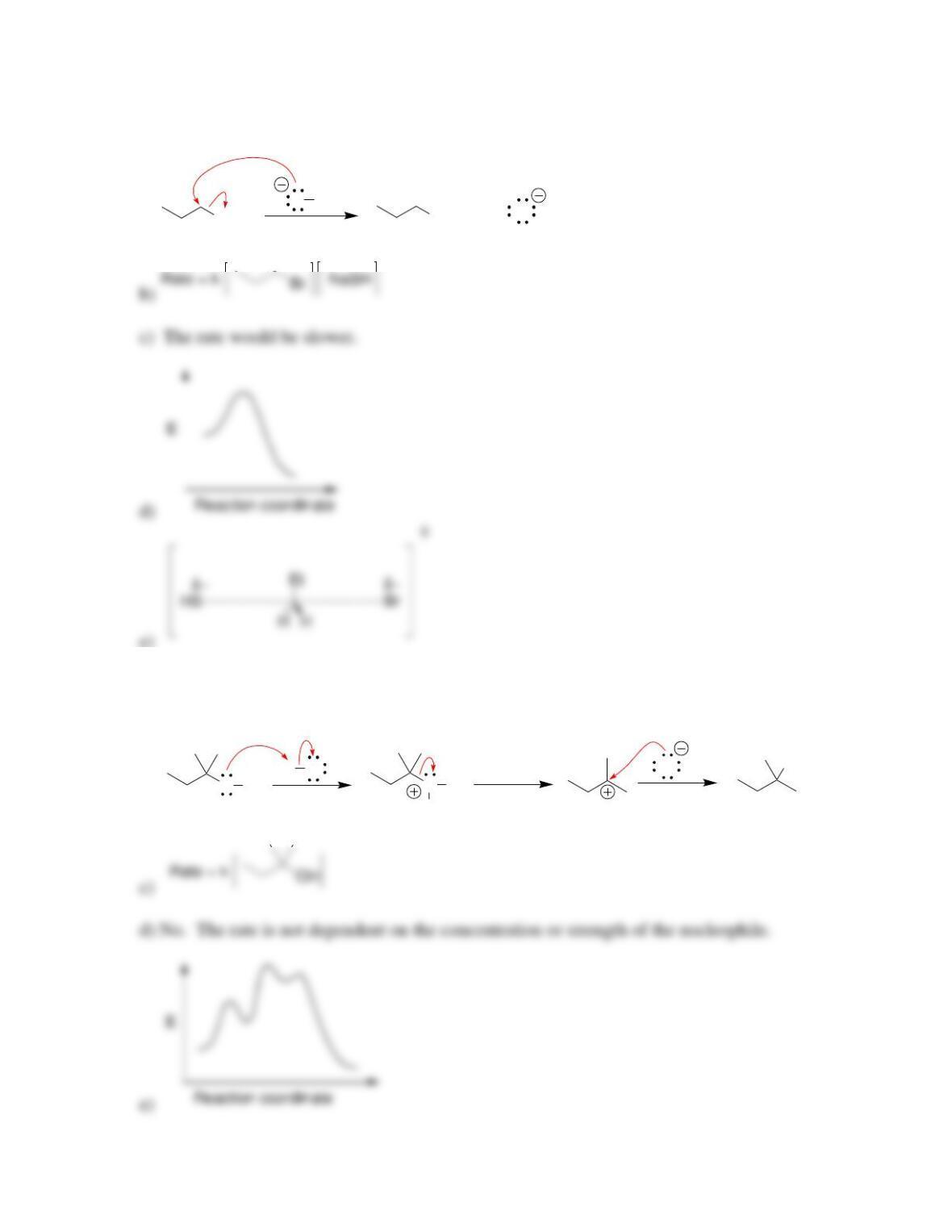
• Evidence for the concerted mechanism, called S
N
2, includes the observation of a
__________-order rate equation. The reaction proceeds with ____________ of
Review of Skills
Follow the instructions below. To verify that your answers are correct, look in your
textbook at the end of Chapter 7. The answers appear in the section entitled SkillBuilder
Review.
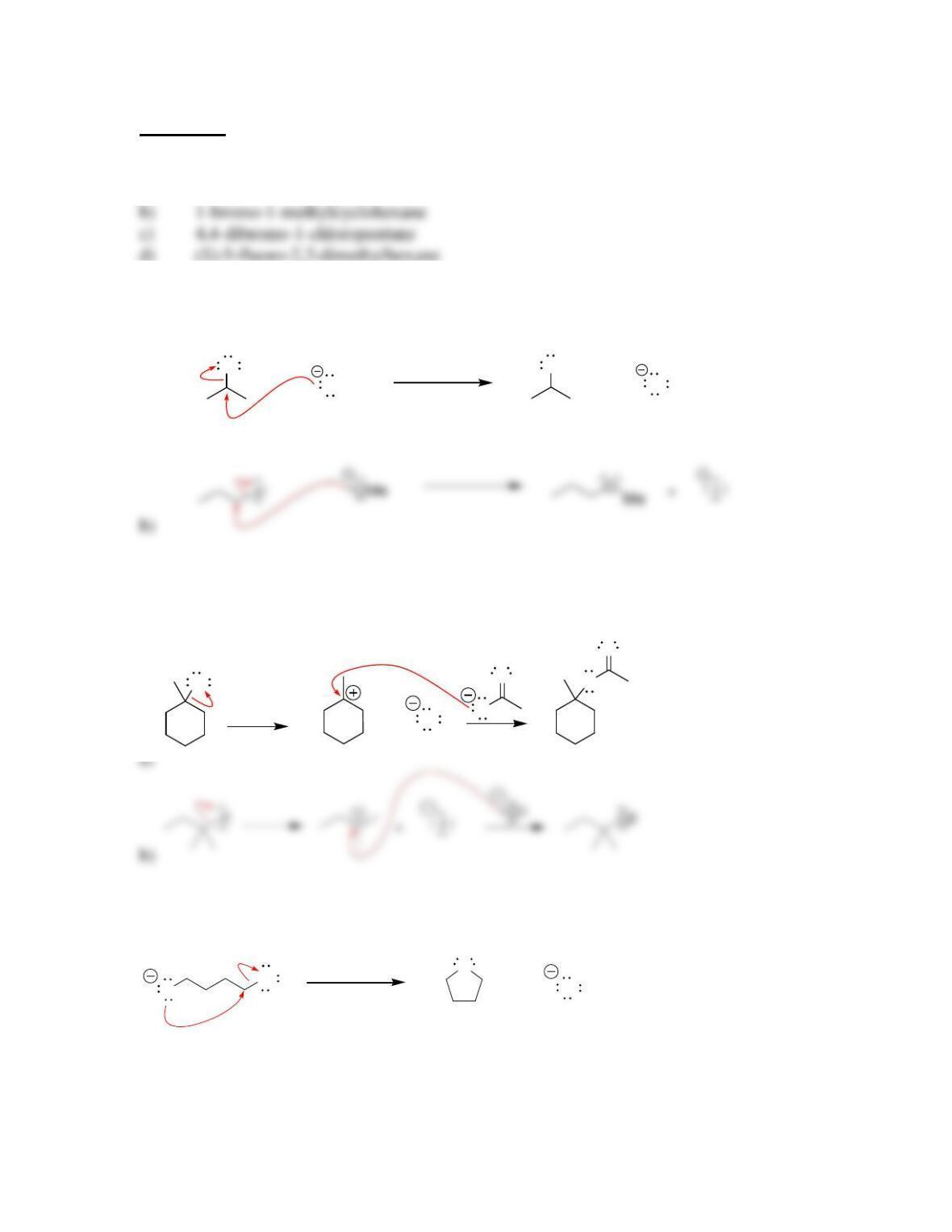
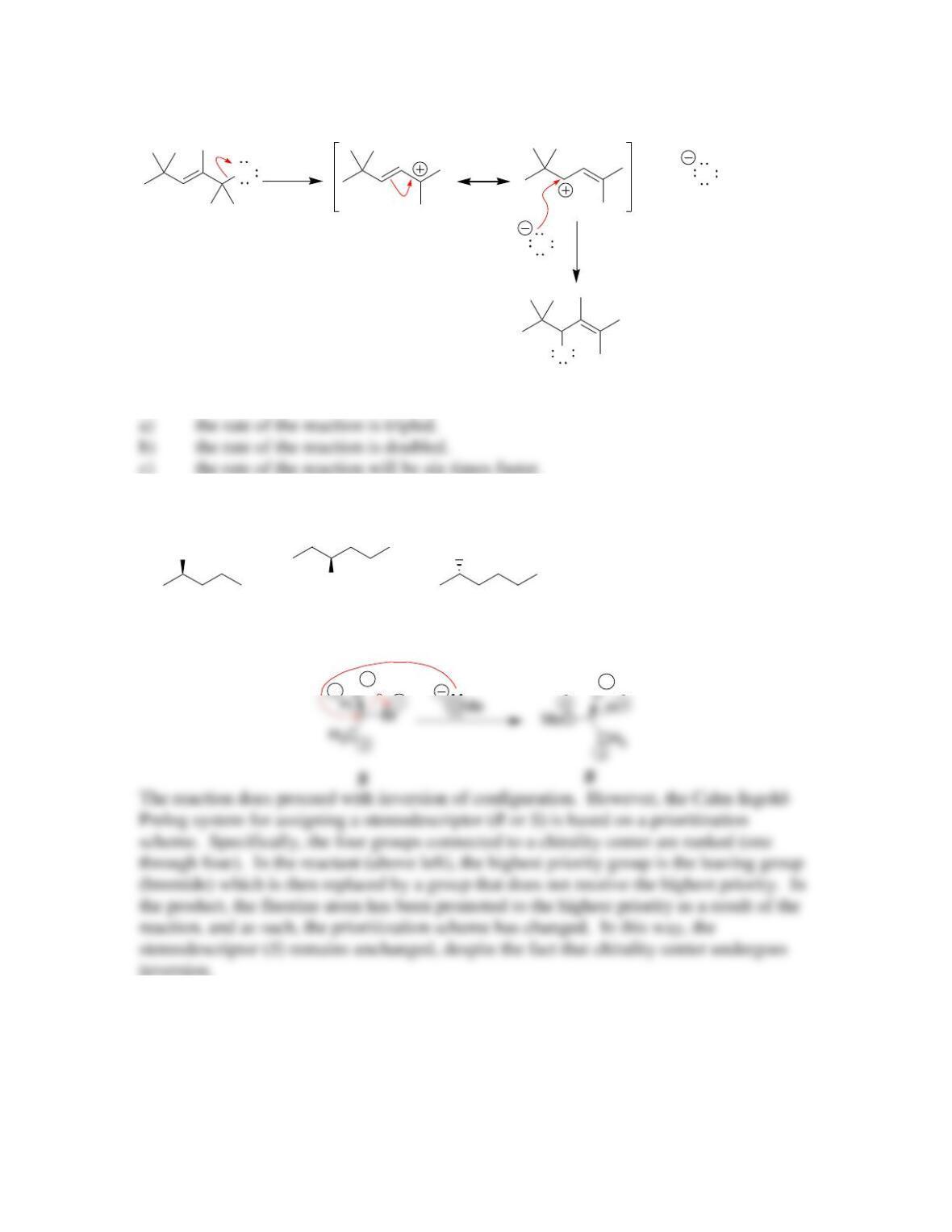
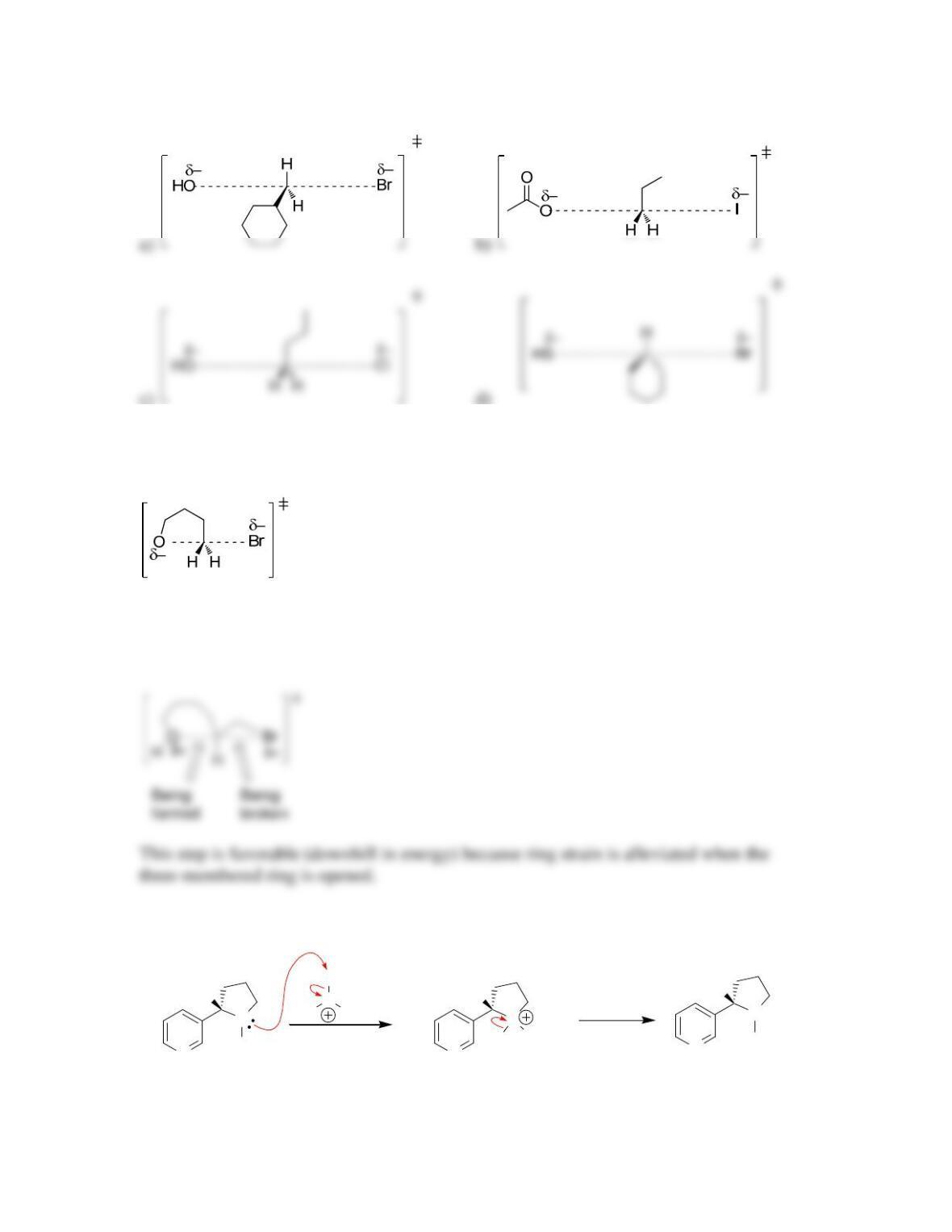
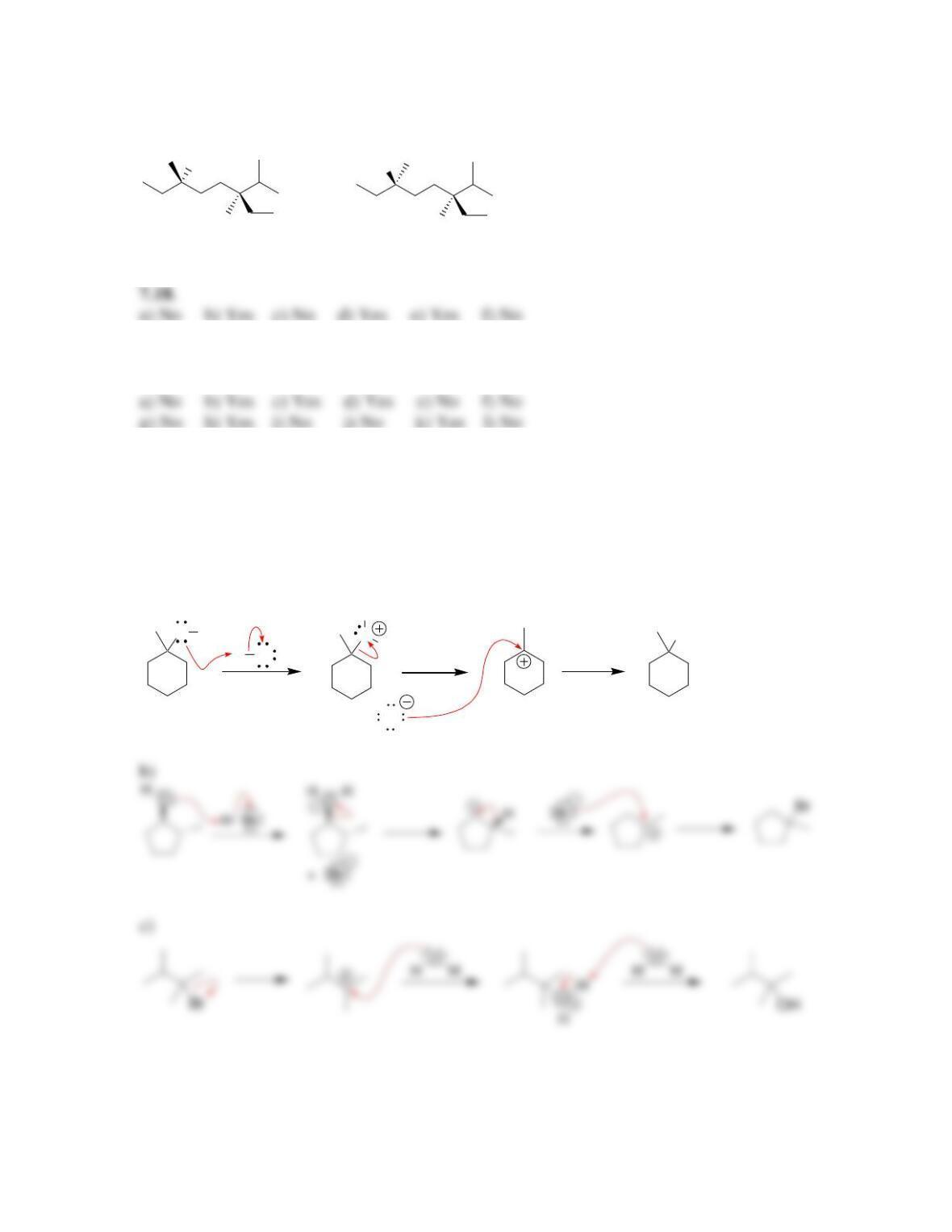
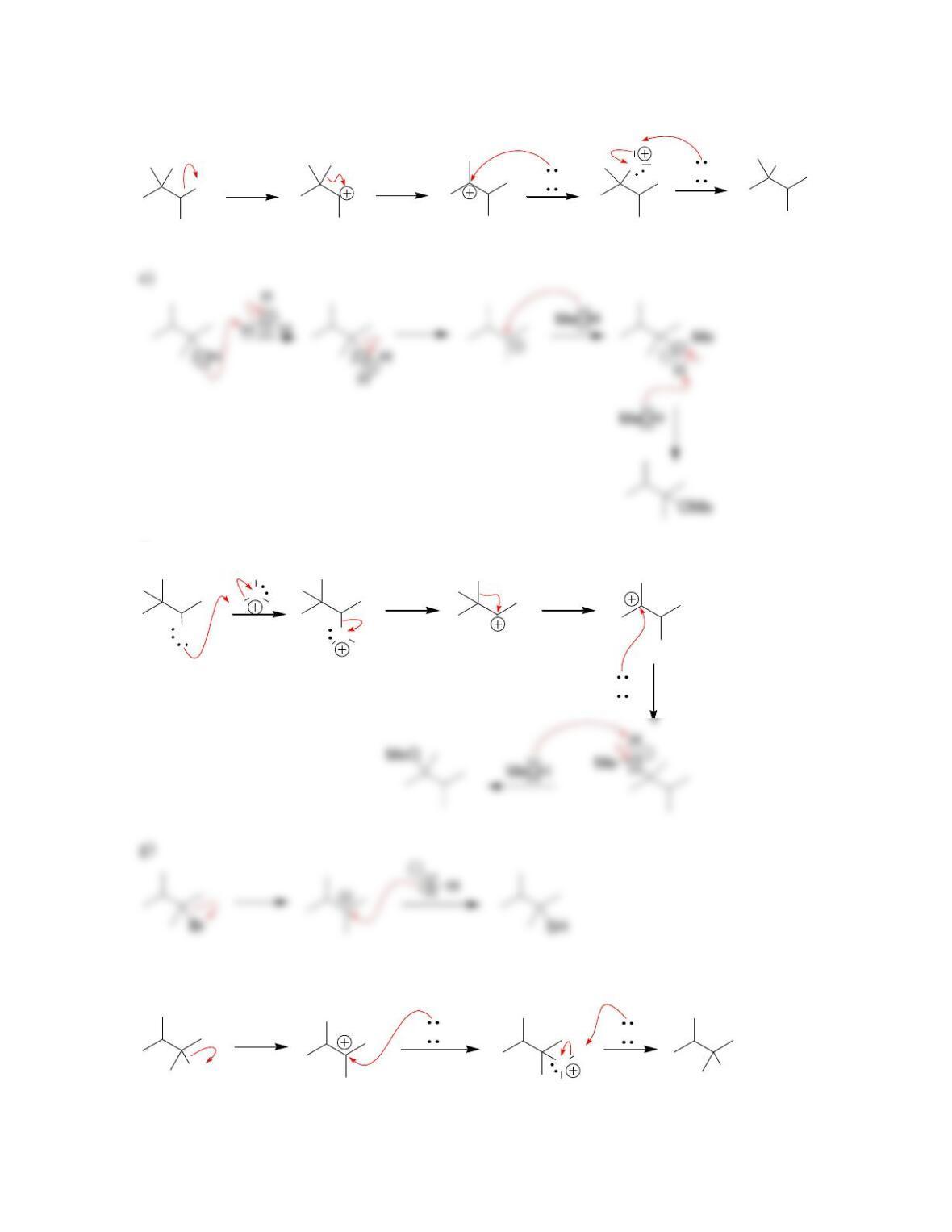
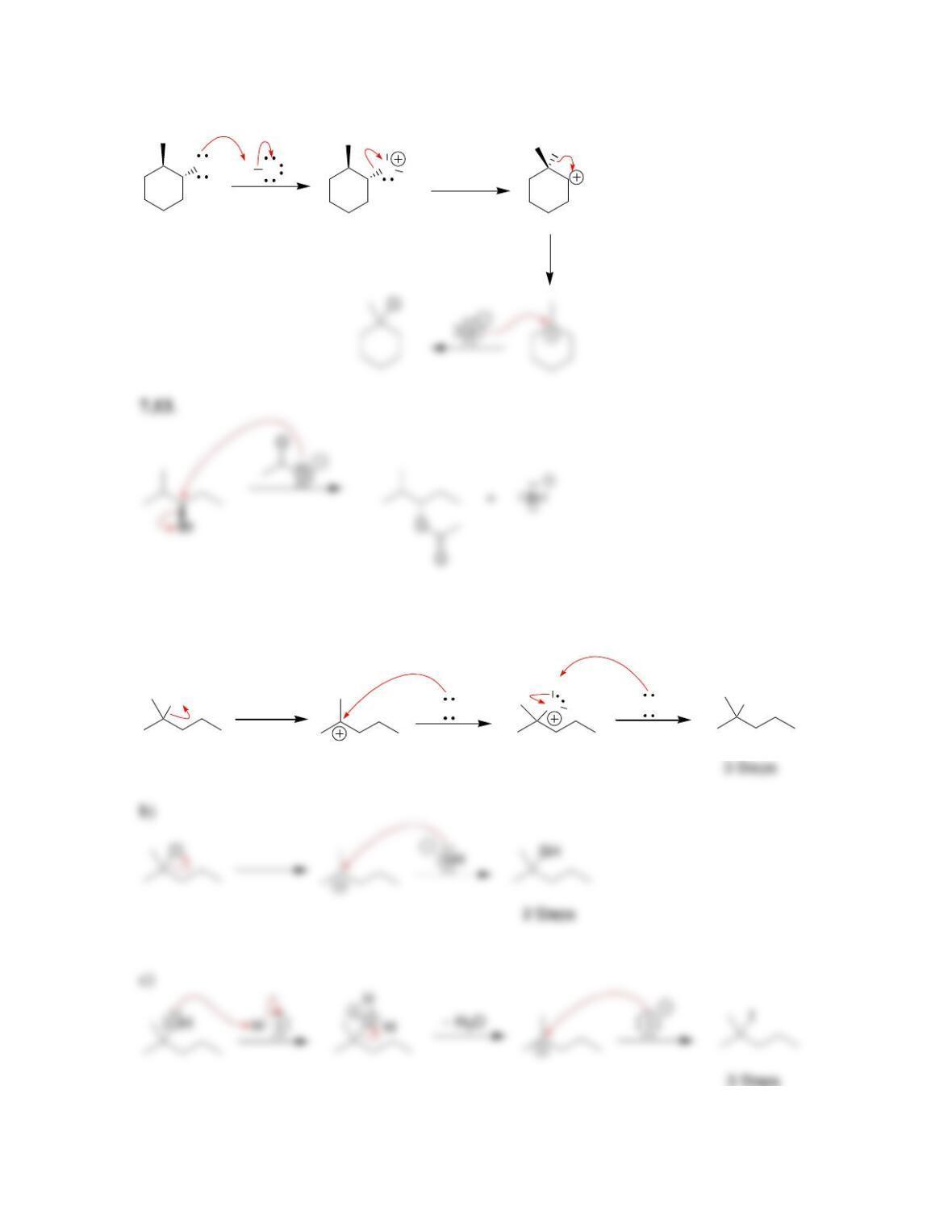
SkillBuilder 7.1 Drawing the Curved Arrows of a Substitution Reaction