CHAPTER 6
111
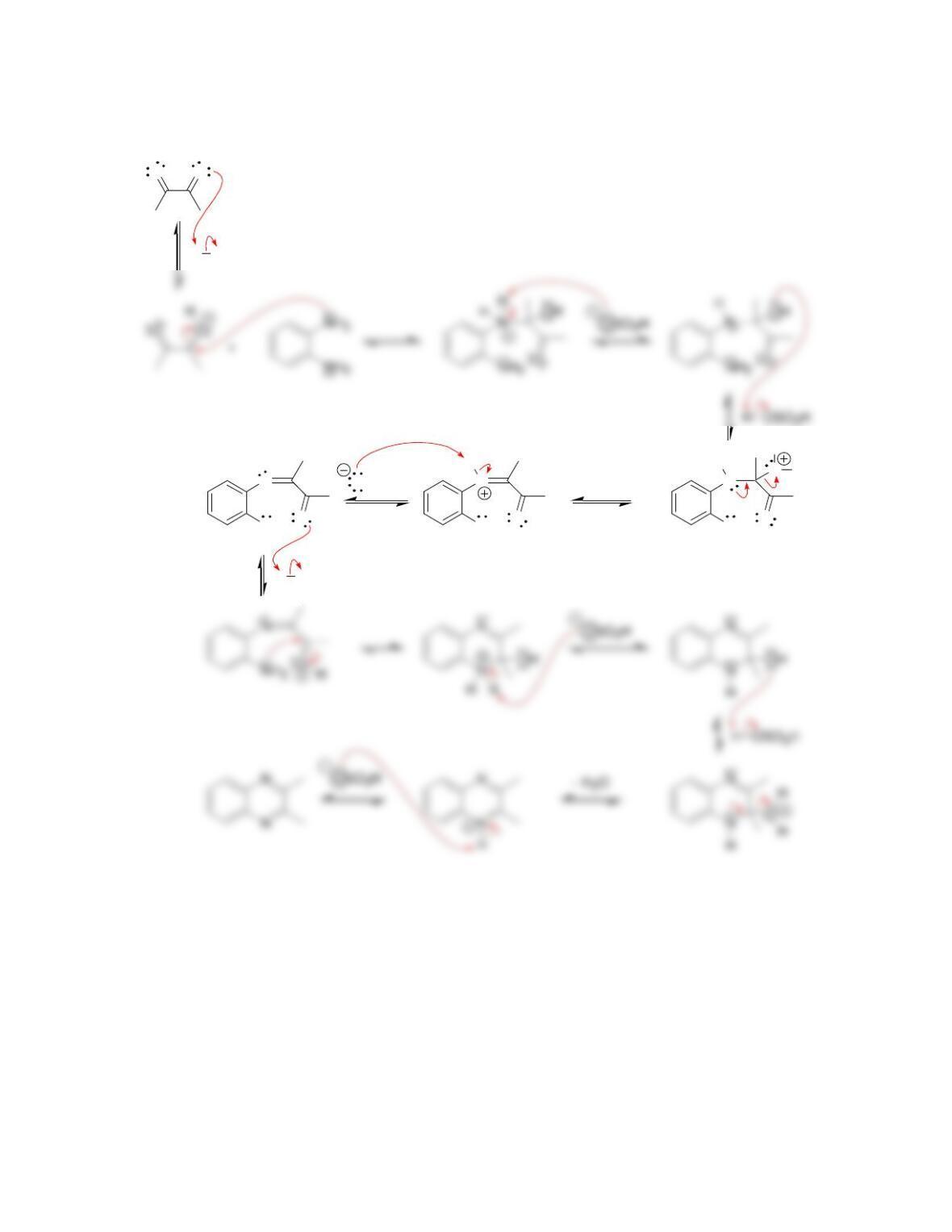
H
6.49.
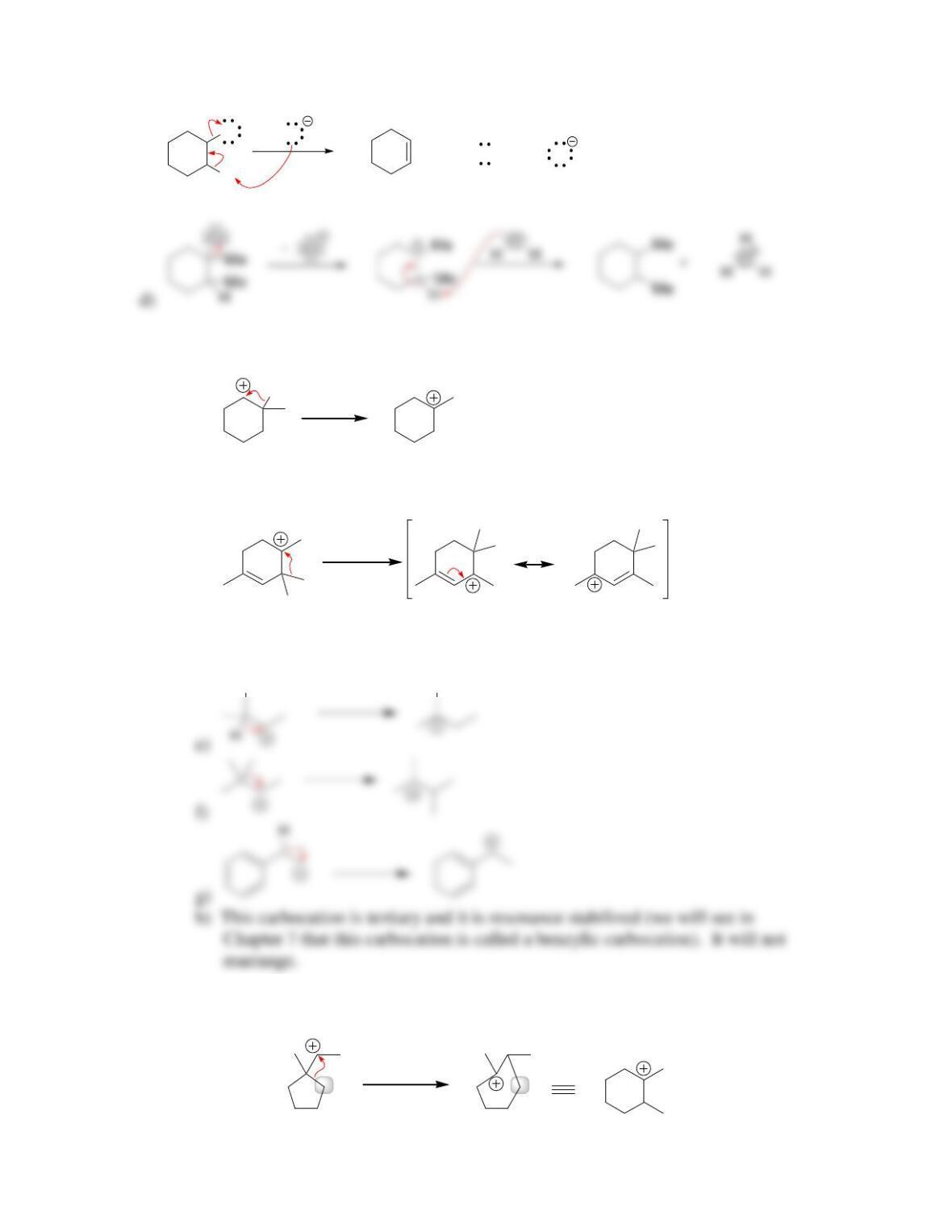
a)
H
3
C
C
HBr
CH
3
C
H
H
OBrOH ++
H
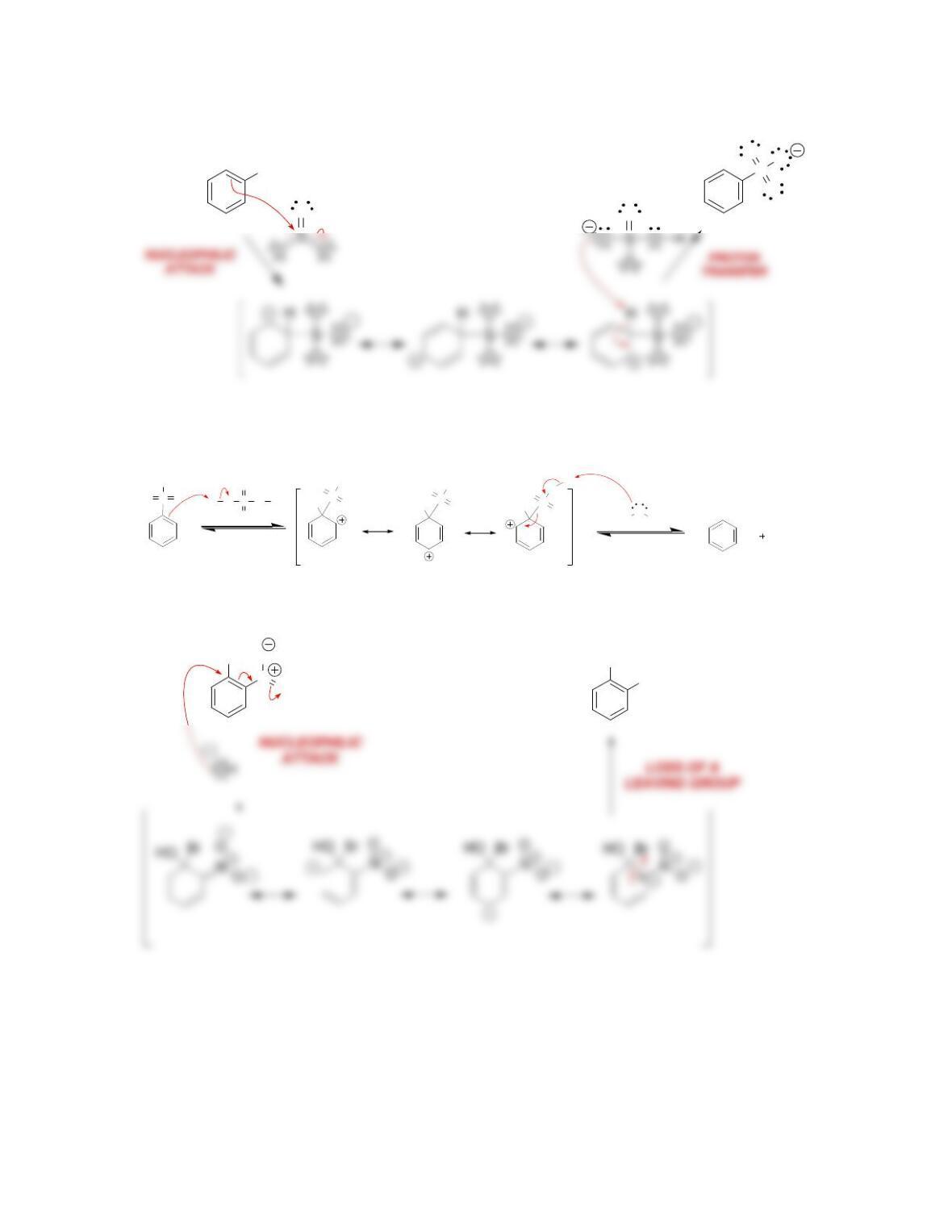
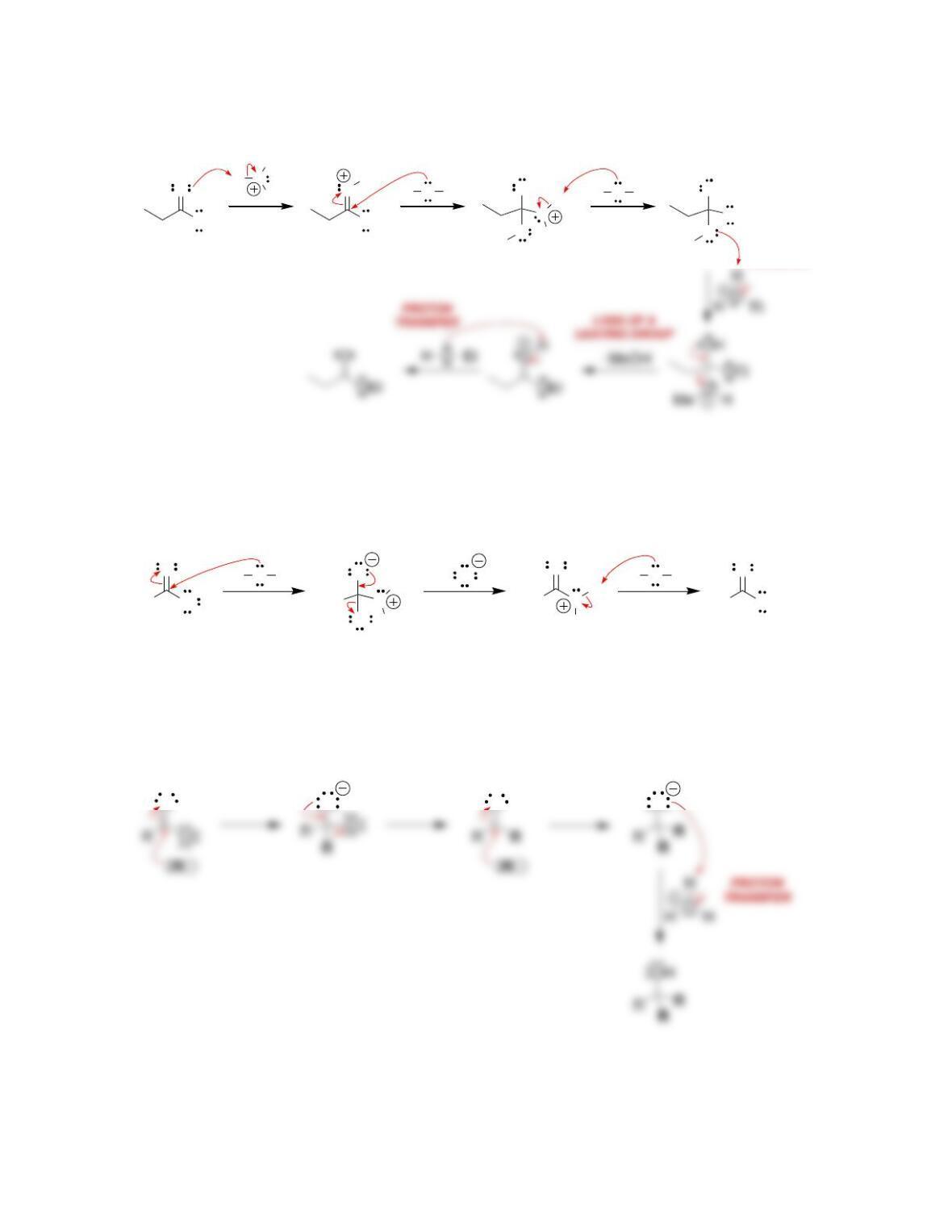
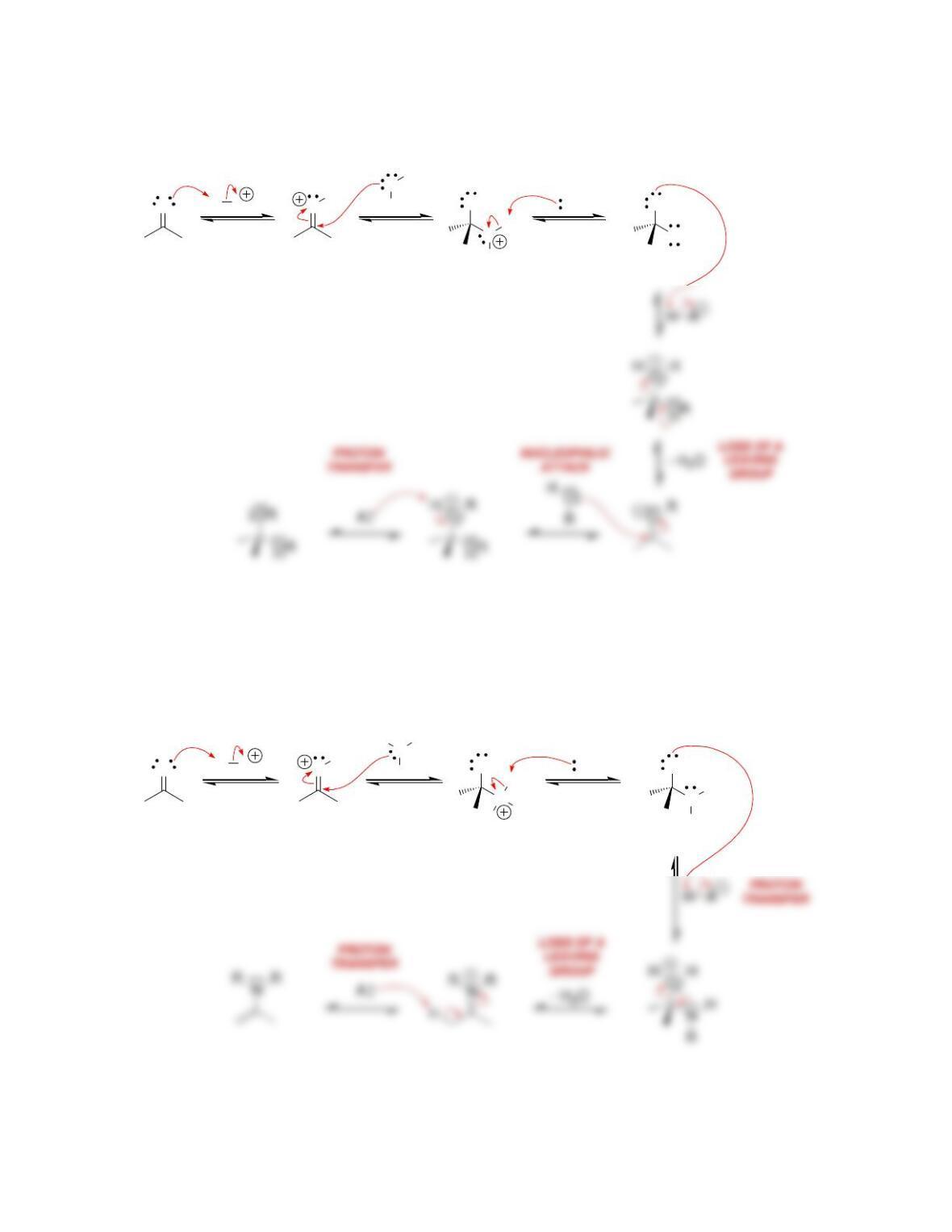
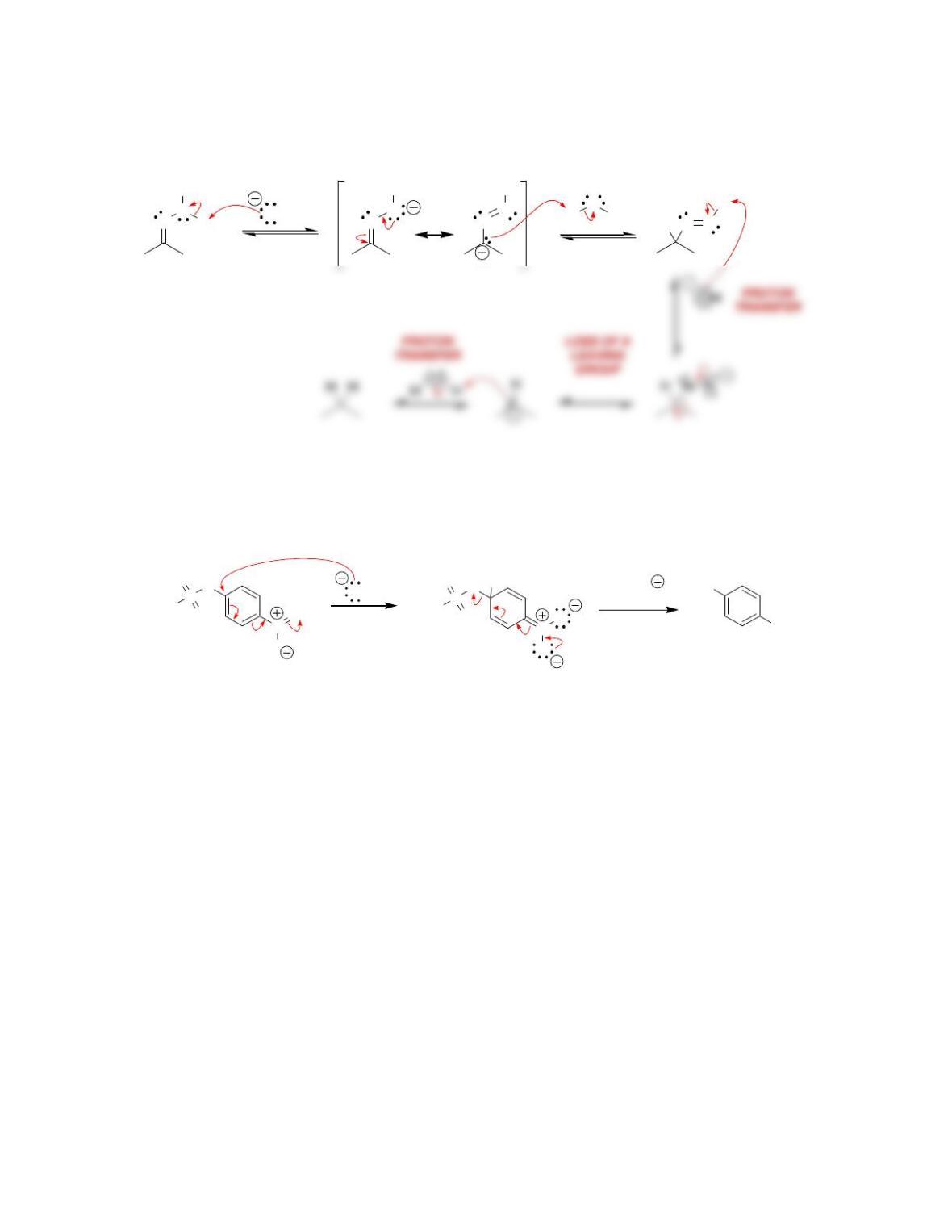
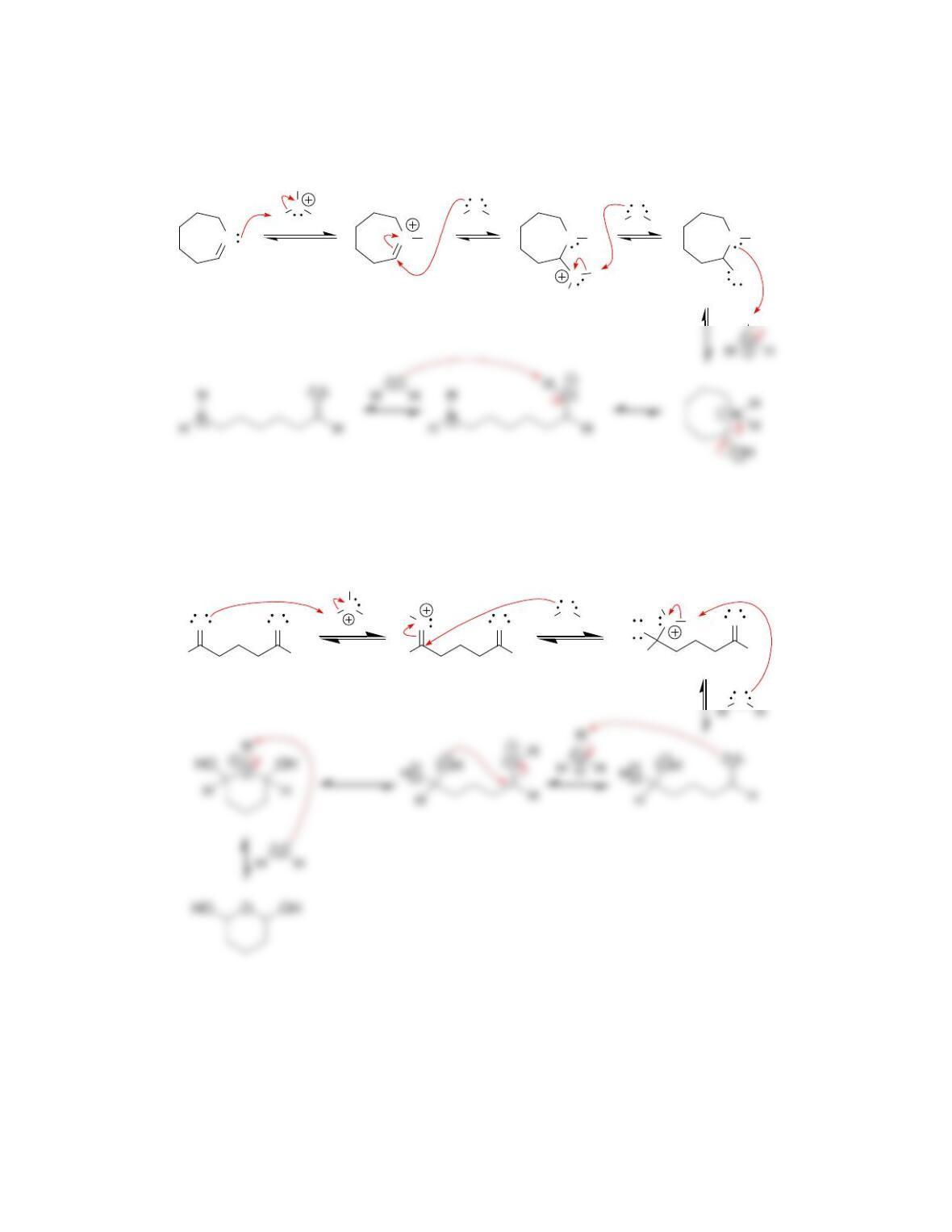
b) Nucleophilic attack and loss of a leaving group.
c) A CH
3
CH
2
—Br is broken, and a CH
3
CH
2
—OH is formed. Using the data in
Table 6.1, ∆H for this reaction is expected to be approximately (285 kJ/mol) –
(381 kJ/mol). The sign of ∆H is therefore predicted to be negative, which means
that the reaction should be exothermic.
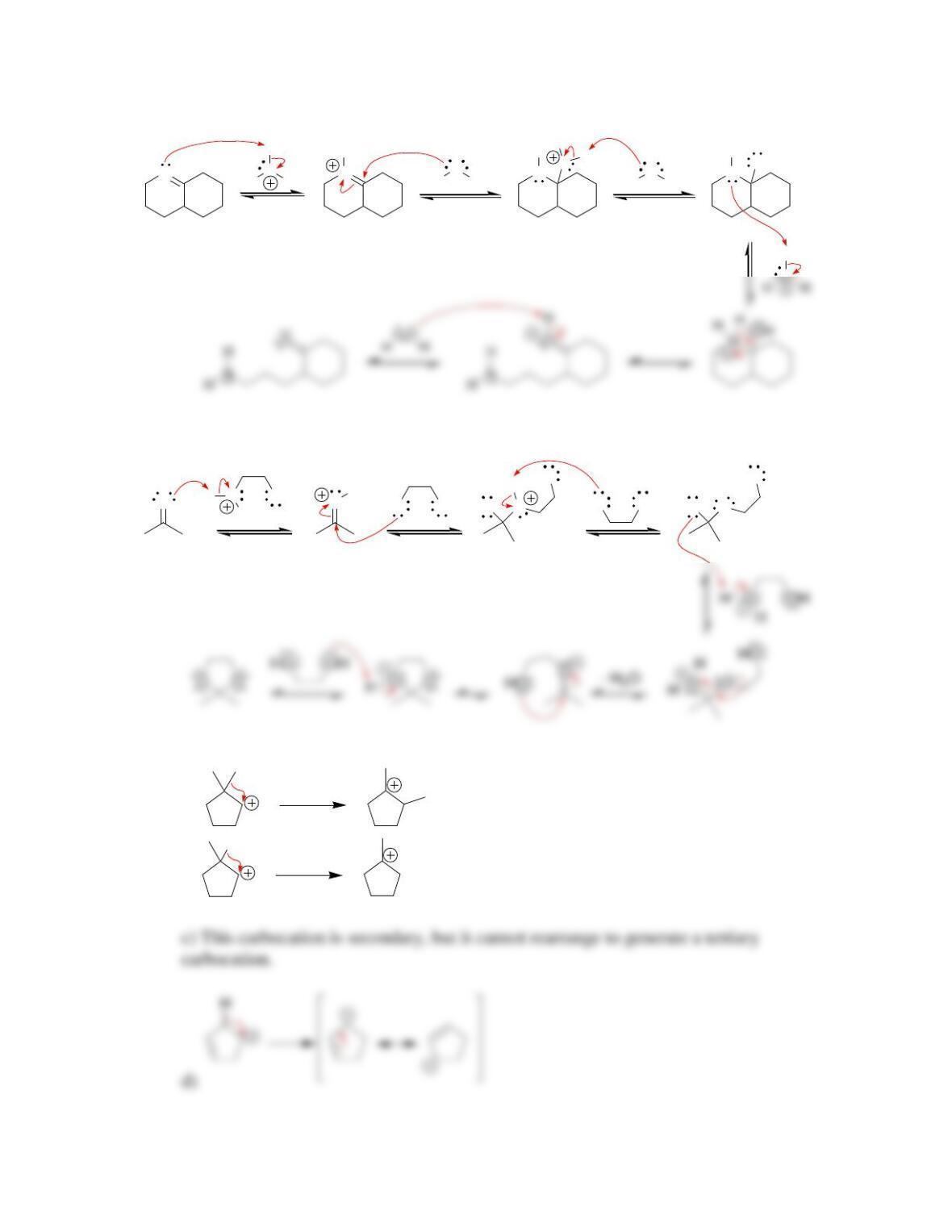
d) Two chemical entities are converted into two chemical entities. Both the
reactants and products are acyclic. Therefore, ∆S for this process is expected to
f) The position of equilibrium is dependent on the sign and value of ∆G. As
mentioned in part e, ∆G is comprised of two terms. The effect of temperature
appears in the second term (-T∆S), which is insignificant because ∆S is
approximately zero. Therefore, an increase (or decrease) in temperature is not
expected to have a significant impact on the position of equilibrium.
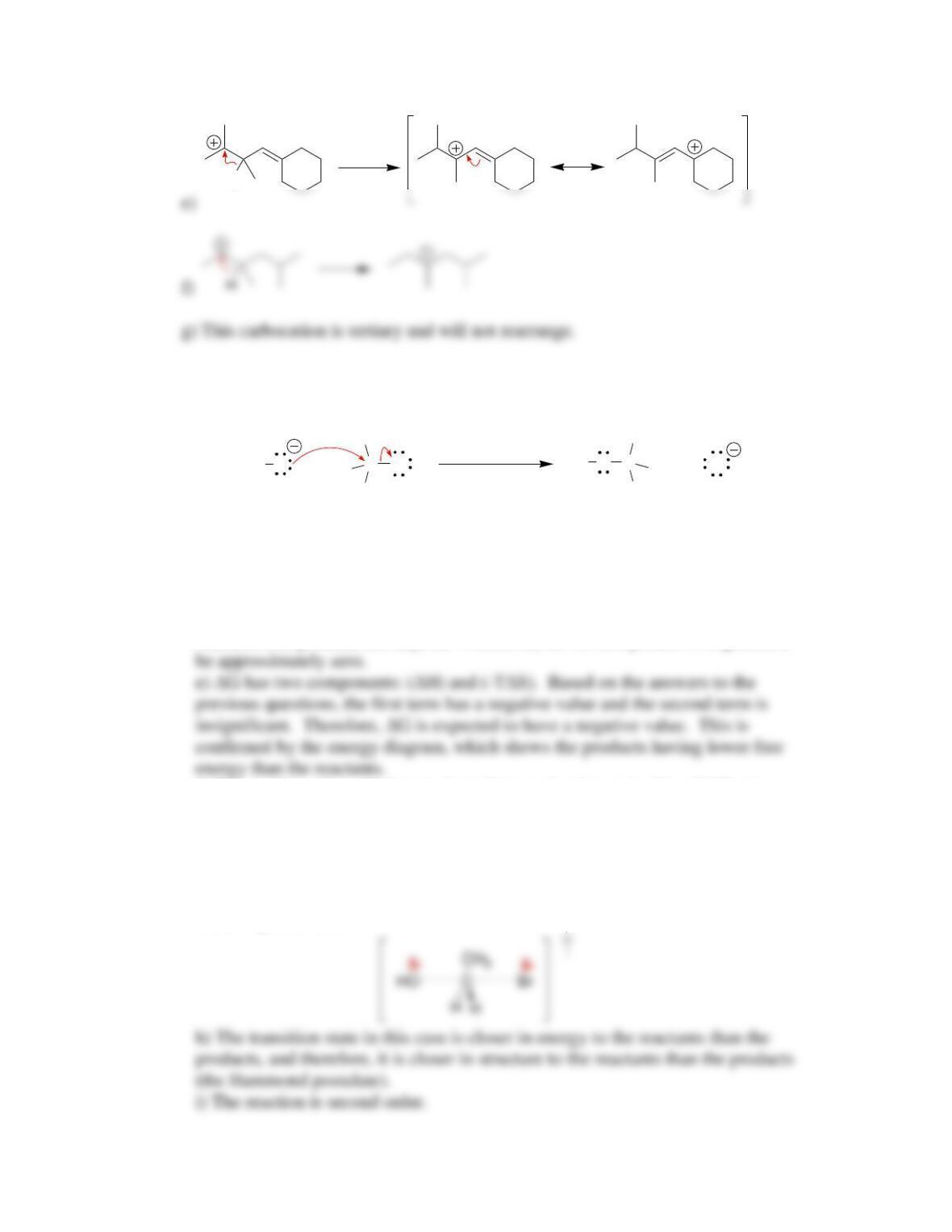
g) This transition state corresponds with the peak of the curve, and has the
following structure: