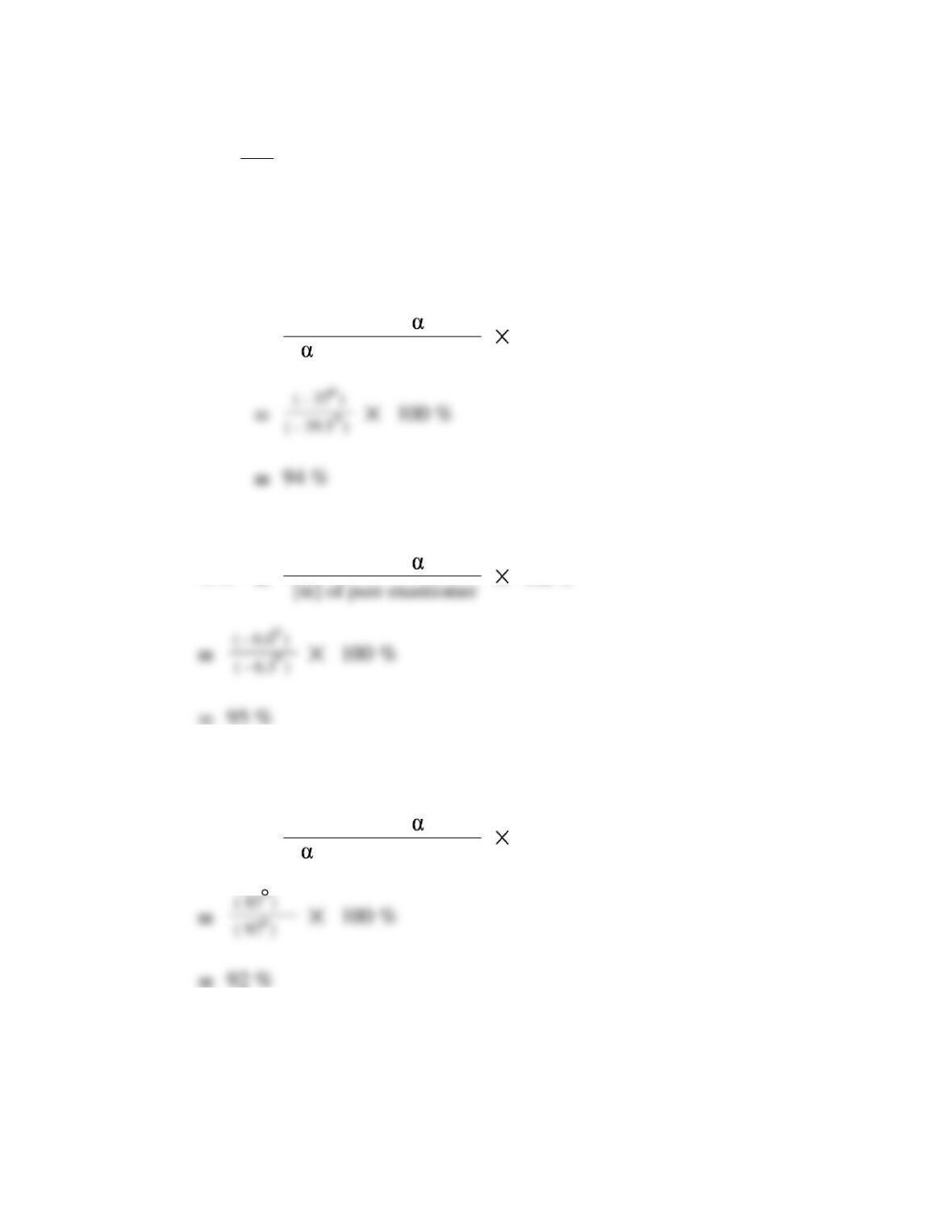
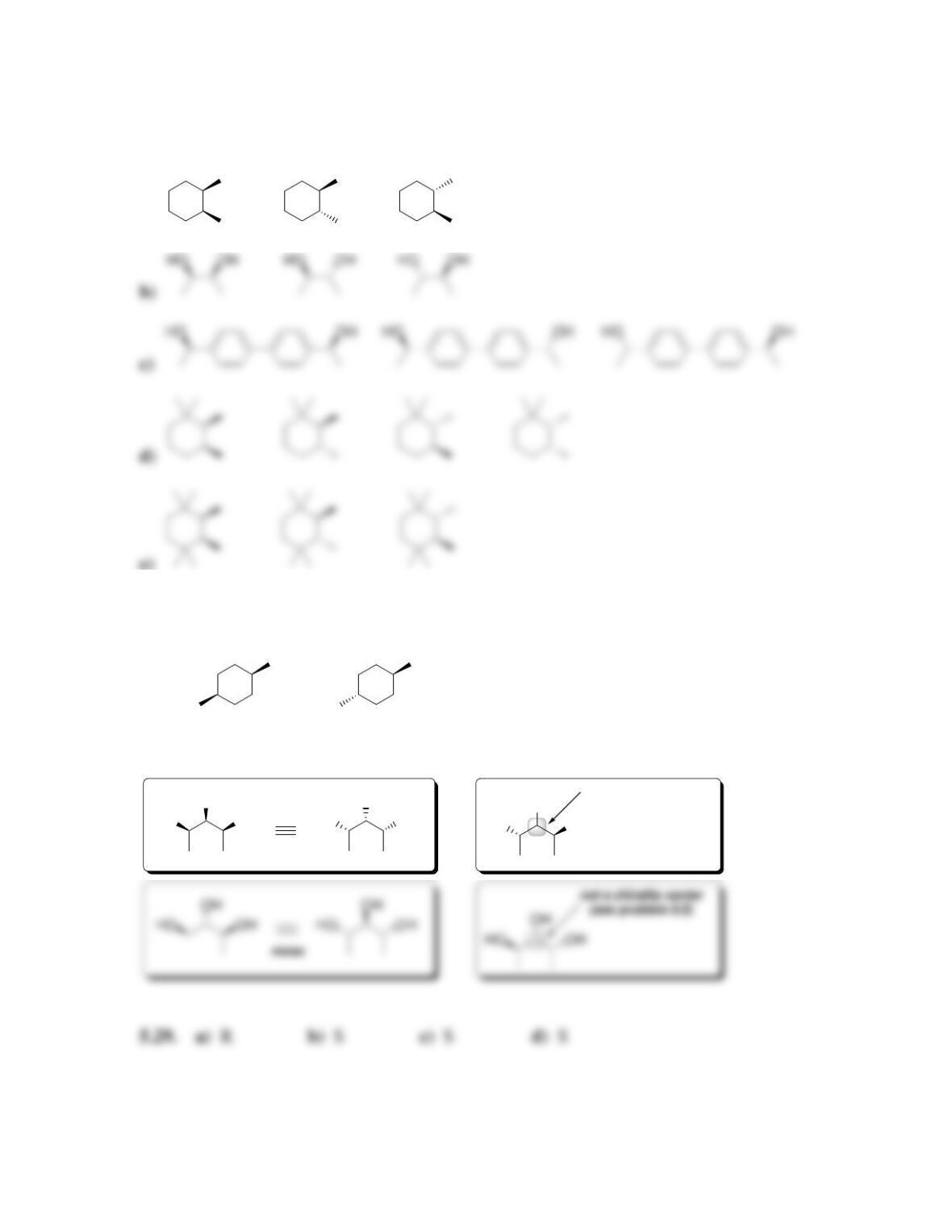
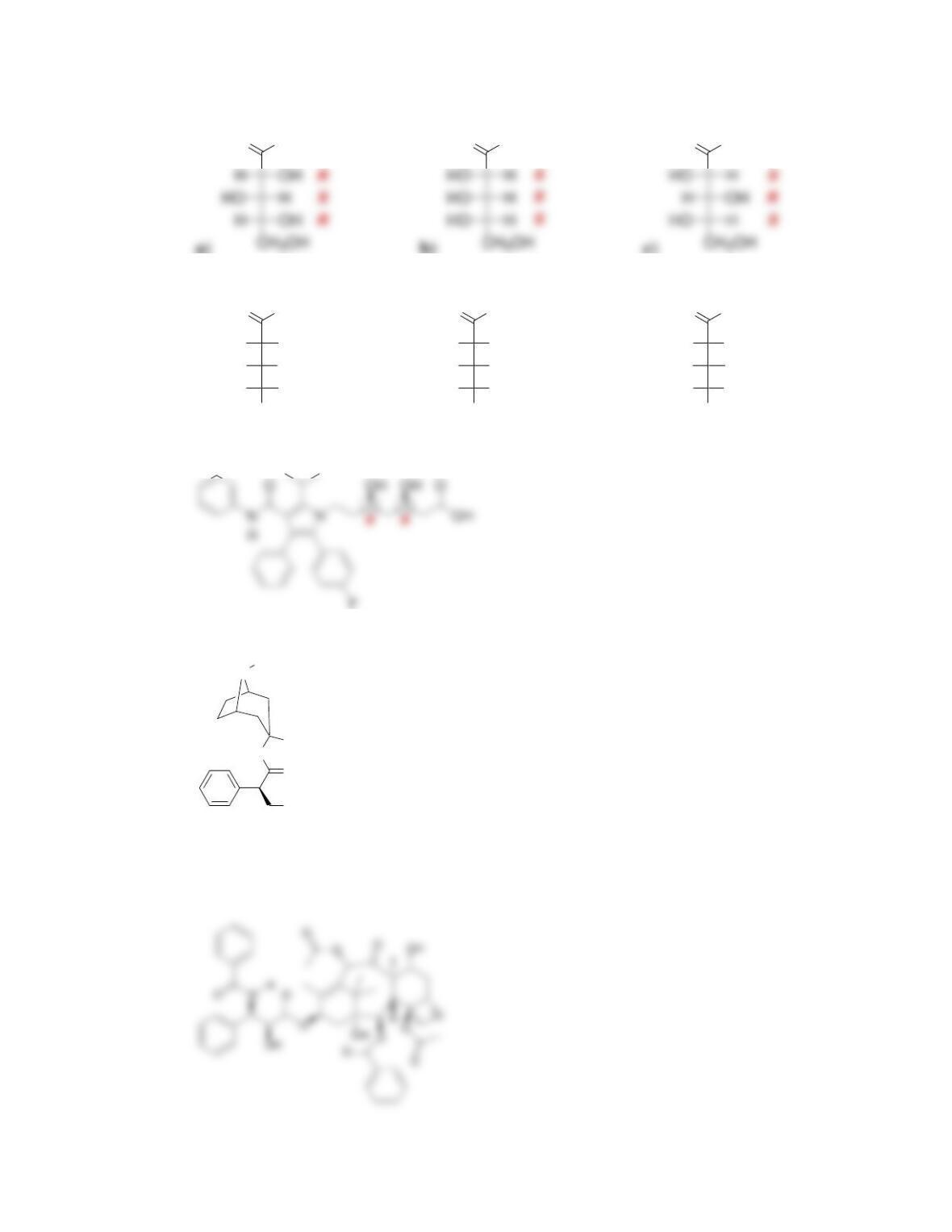
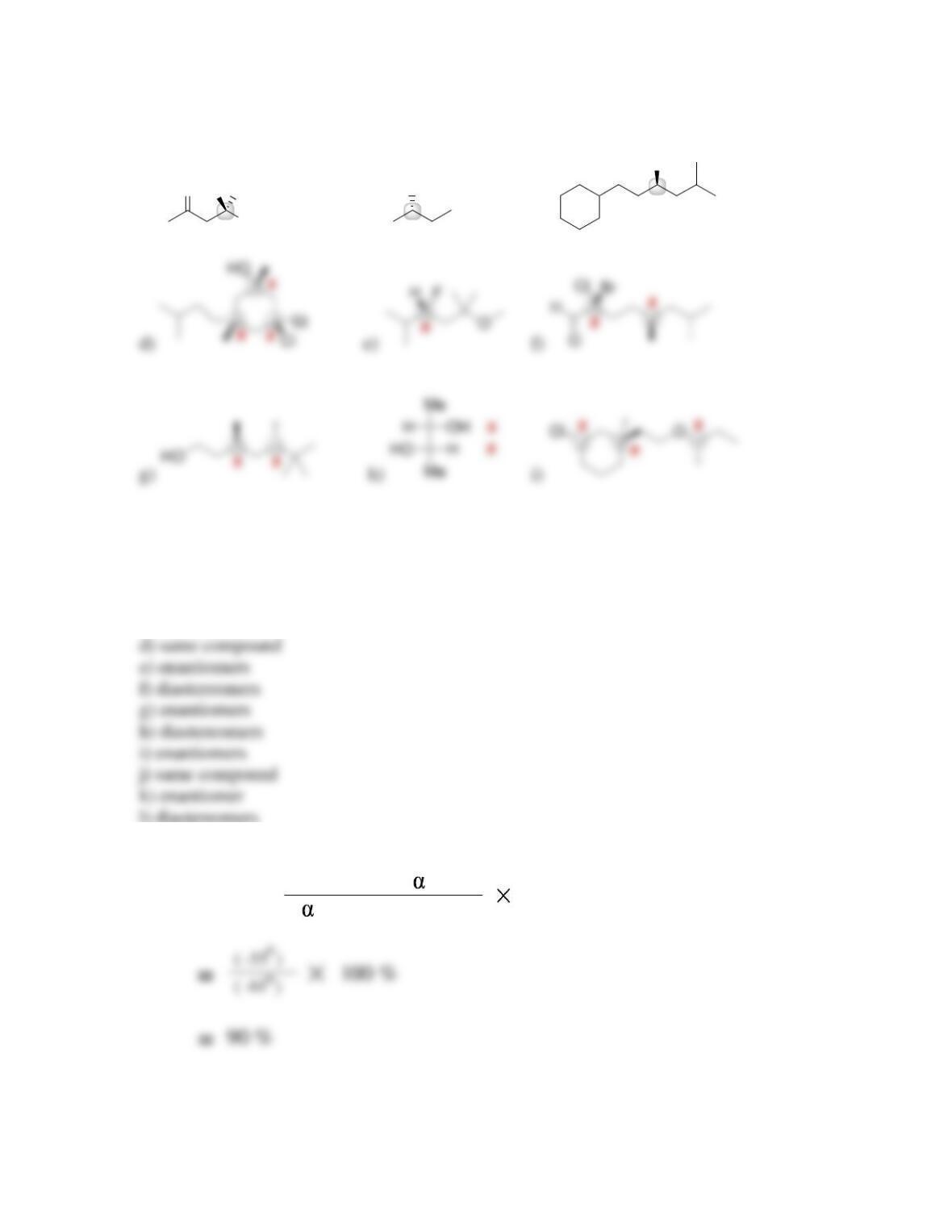
Chapter 5
Stereoisomerism
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 5. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• ______isomers have the same connectivity of atoms but differ in their spatial
arrangement.
• Chiral objects are not superimposable on their ____________________. The
most common source of molecular chirality is the presence of a
_______________, a carbon atom bearing ______ different groups.
• A compound with one chirality center will have one non-superimposable mirror
image, called its _______________.
Review of Skills
Fill in the blanks and empty boxes below. To verify that your answers are correct, look
in your textbook at the end of Chapter 5. The answers appear in the section entitled
SkillBuilder Review.
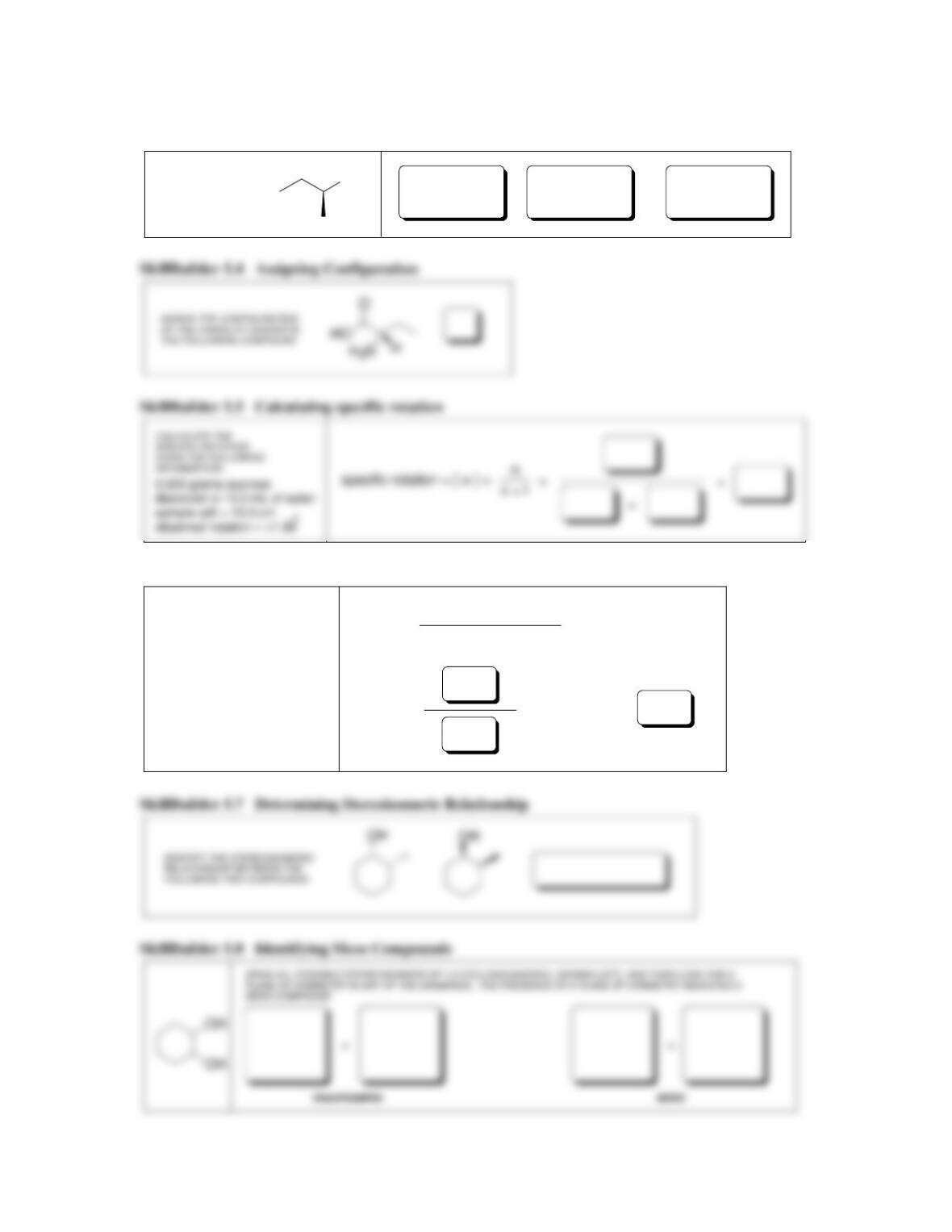
SkillBuilder 5.1 Identifying cis-trans Stereoisomerism
ASSIGN THE CONFIGURATION
OF THE FOLLOWING DOUBLE
BOND AS CIS OR TRANS