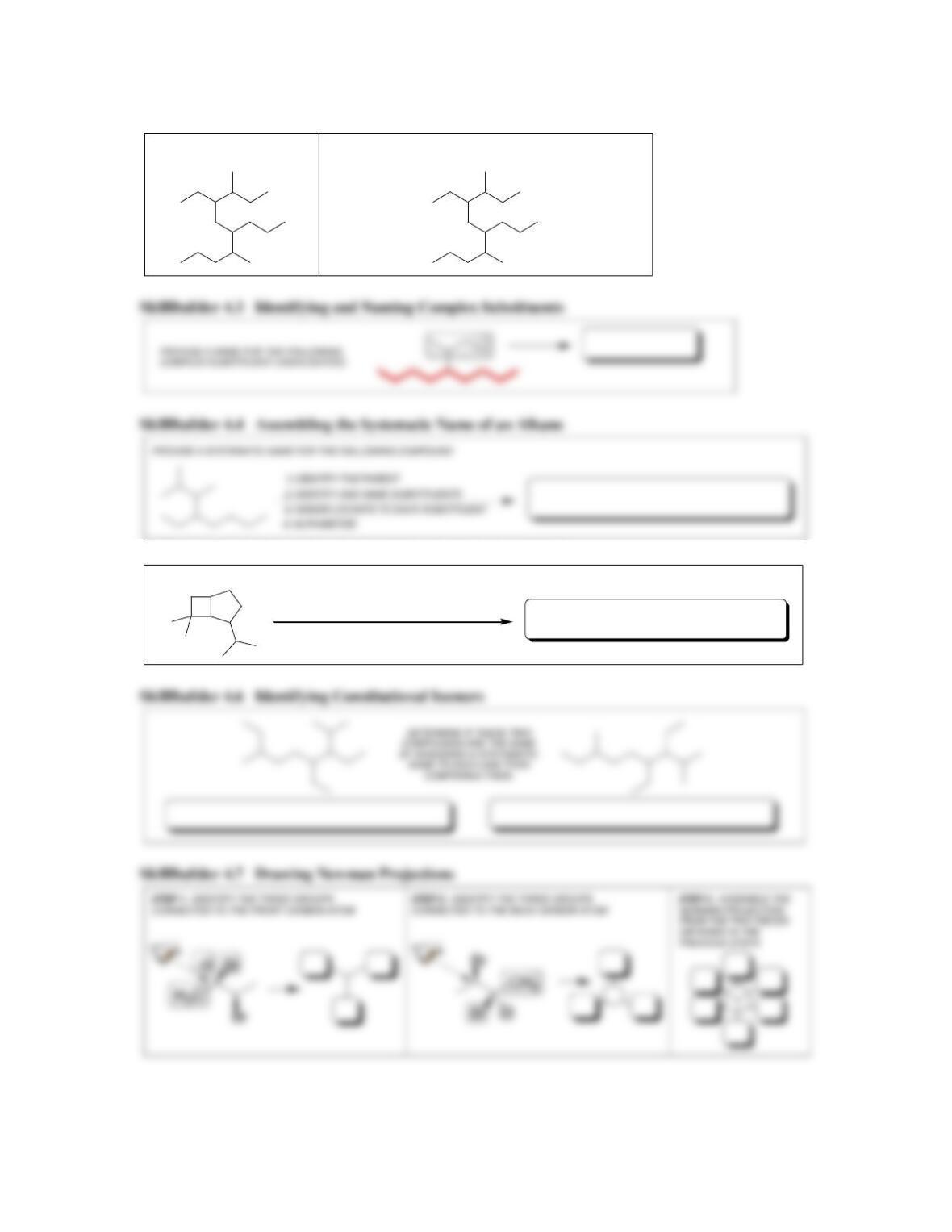
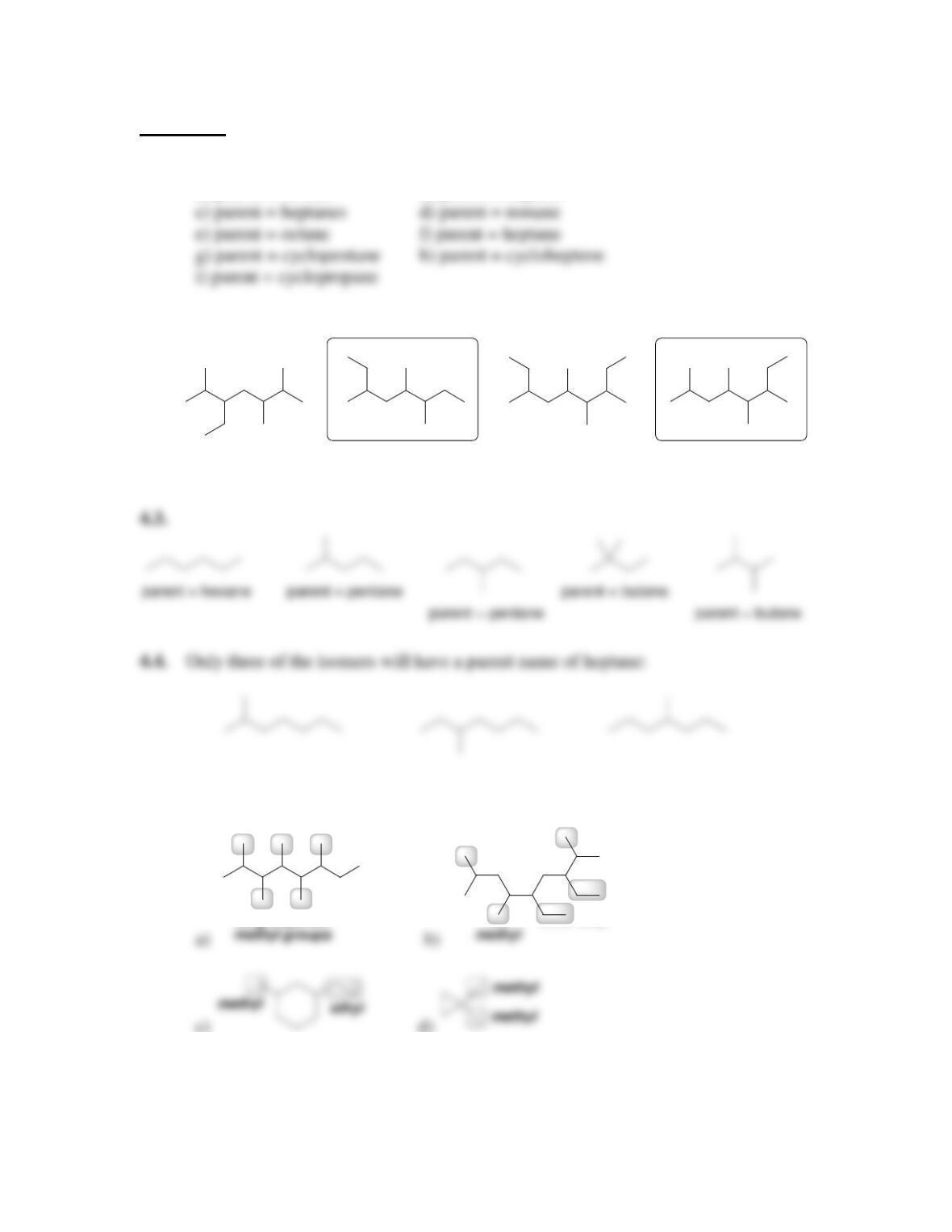
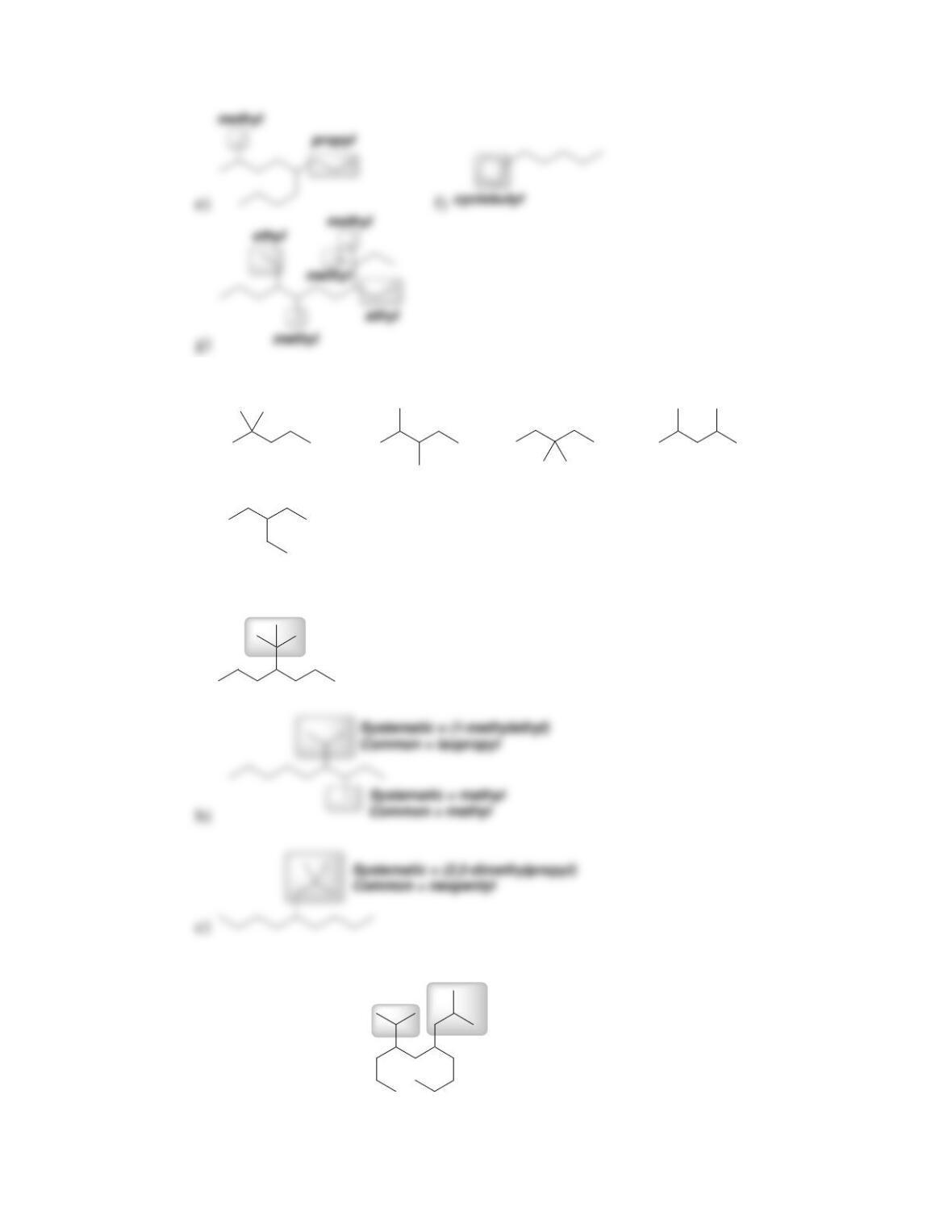
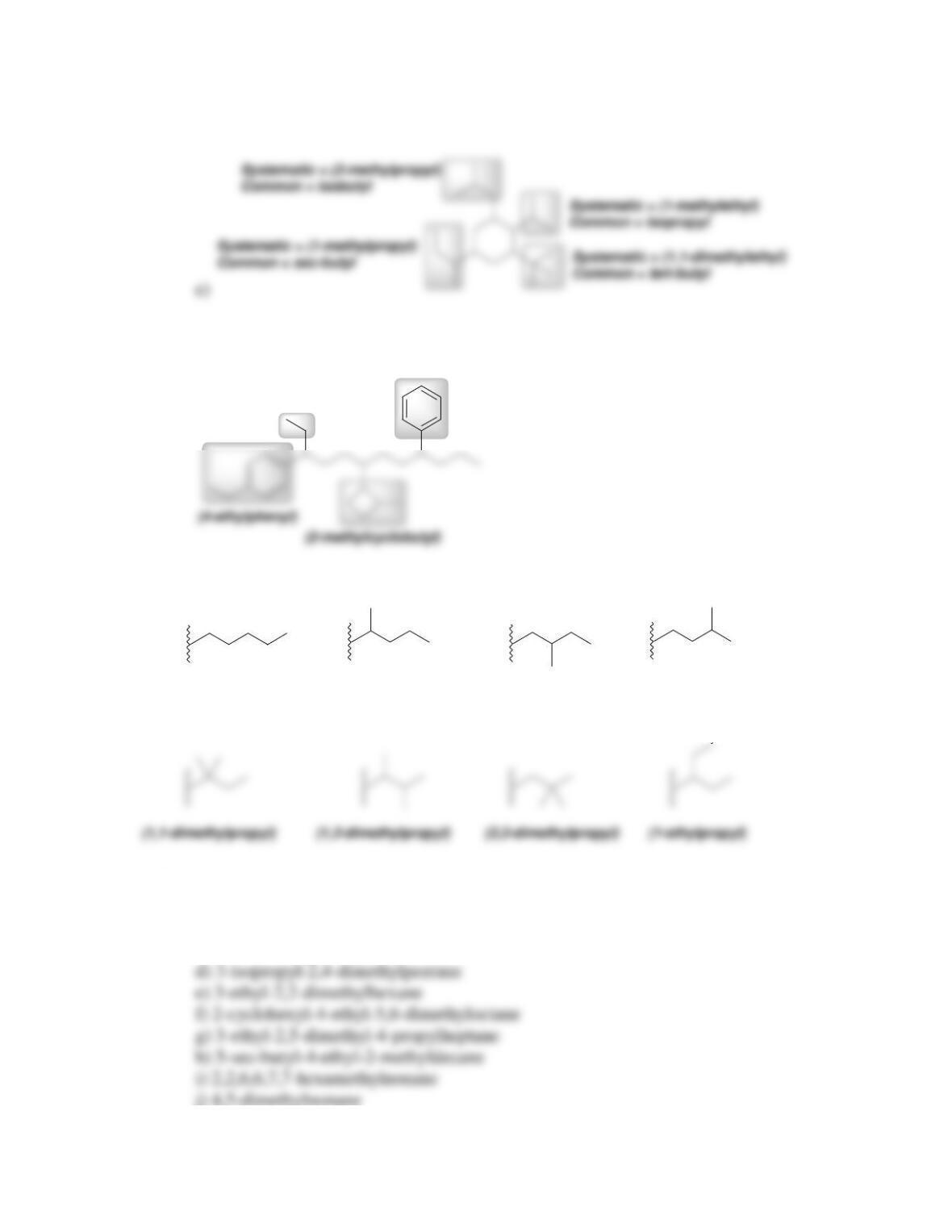
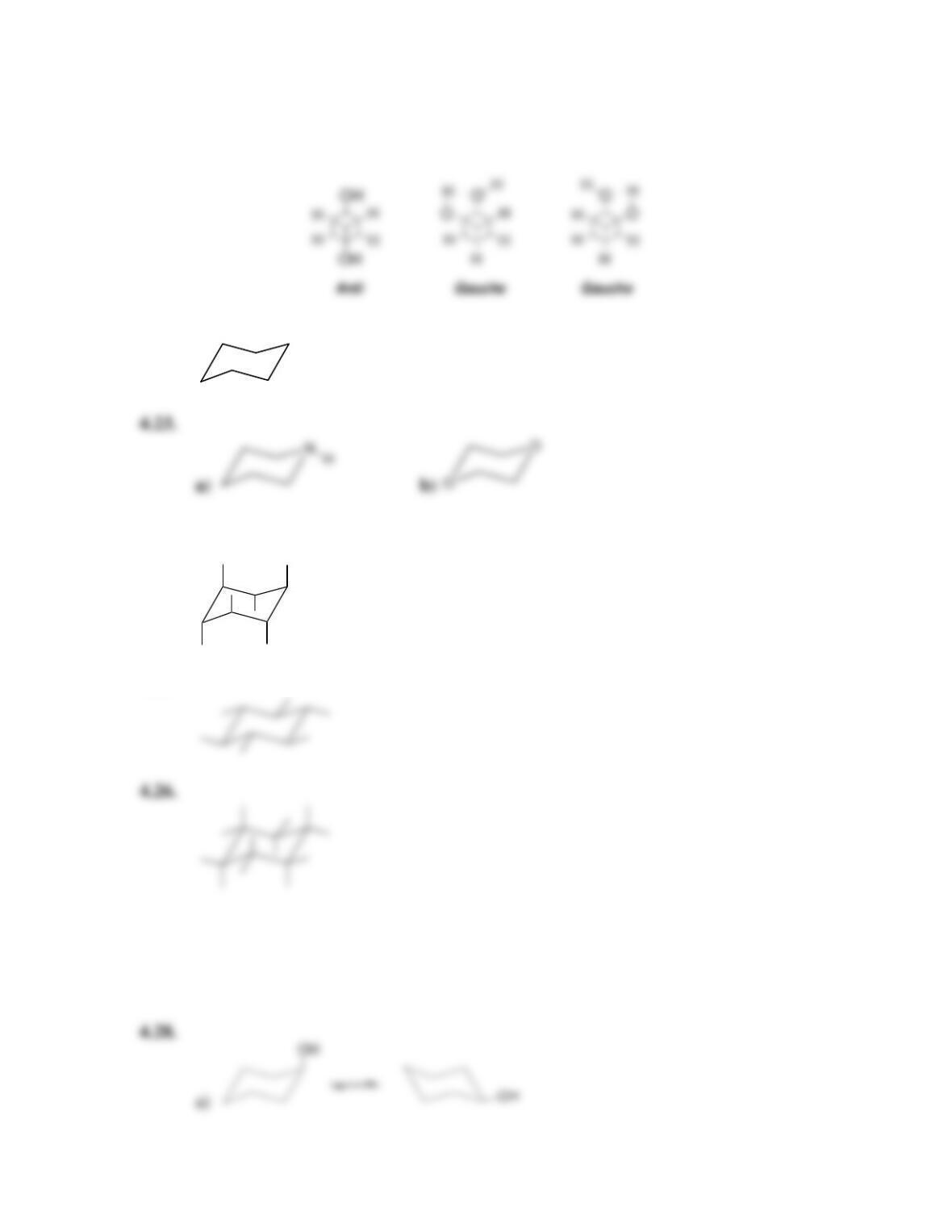
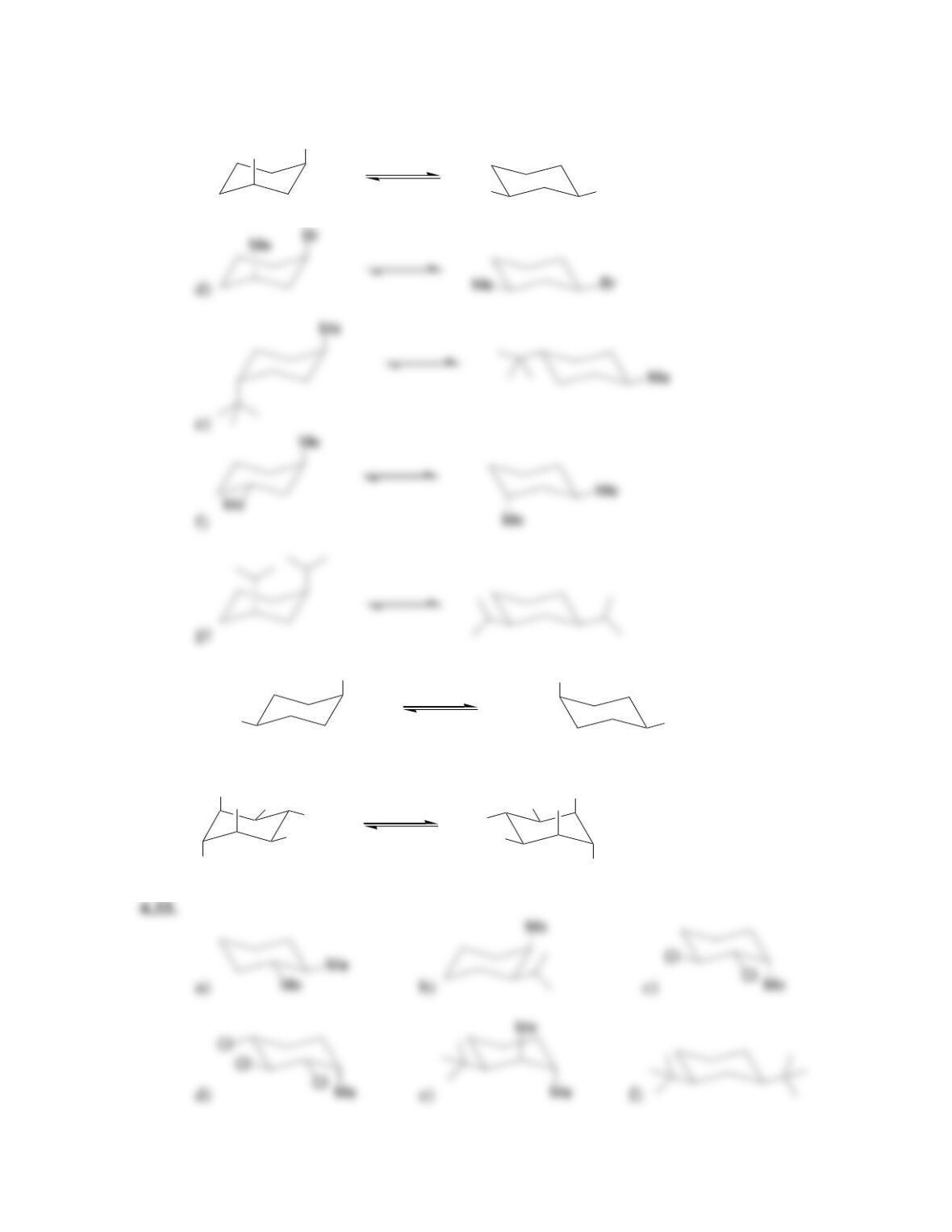
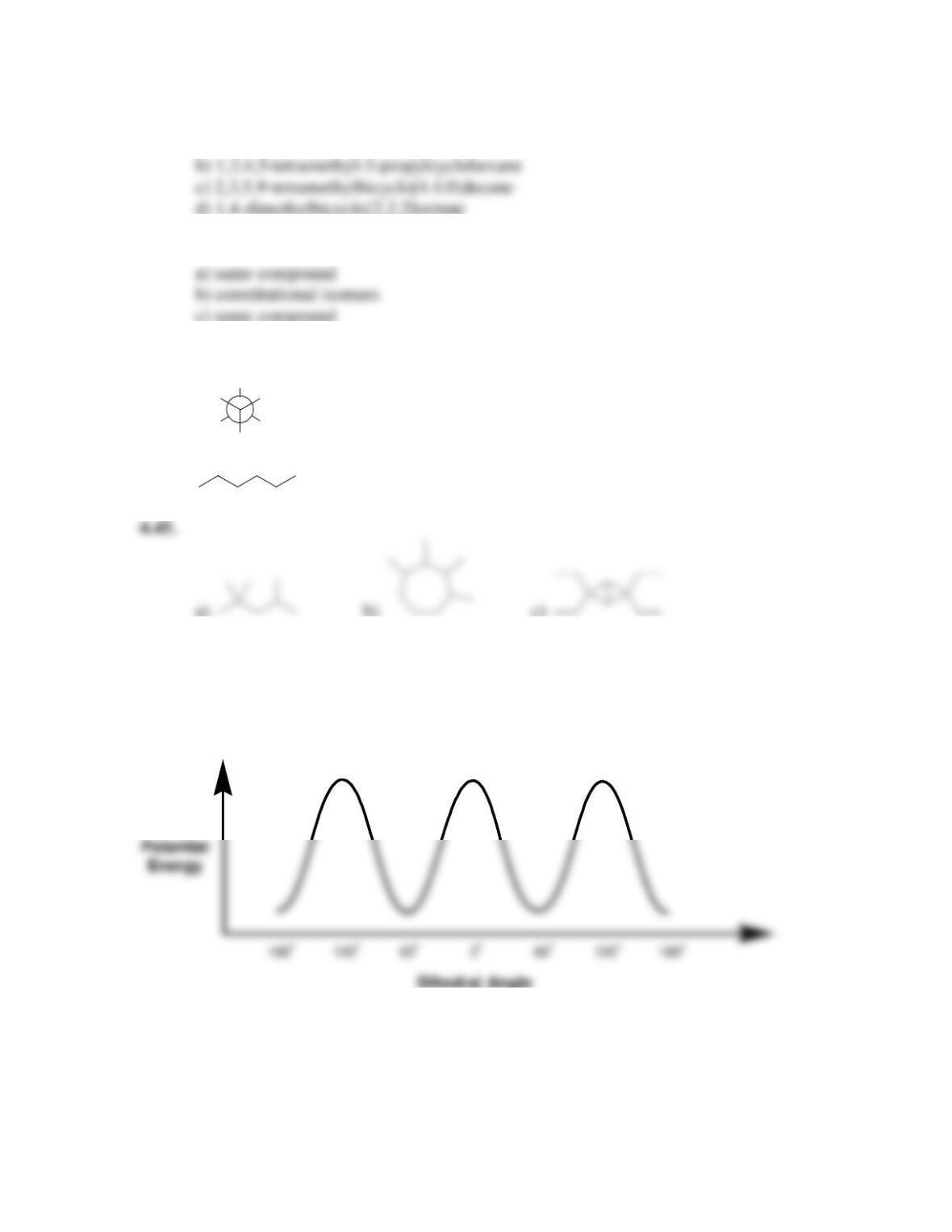
68
CHAPTER 4
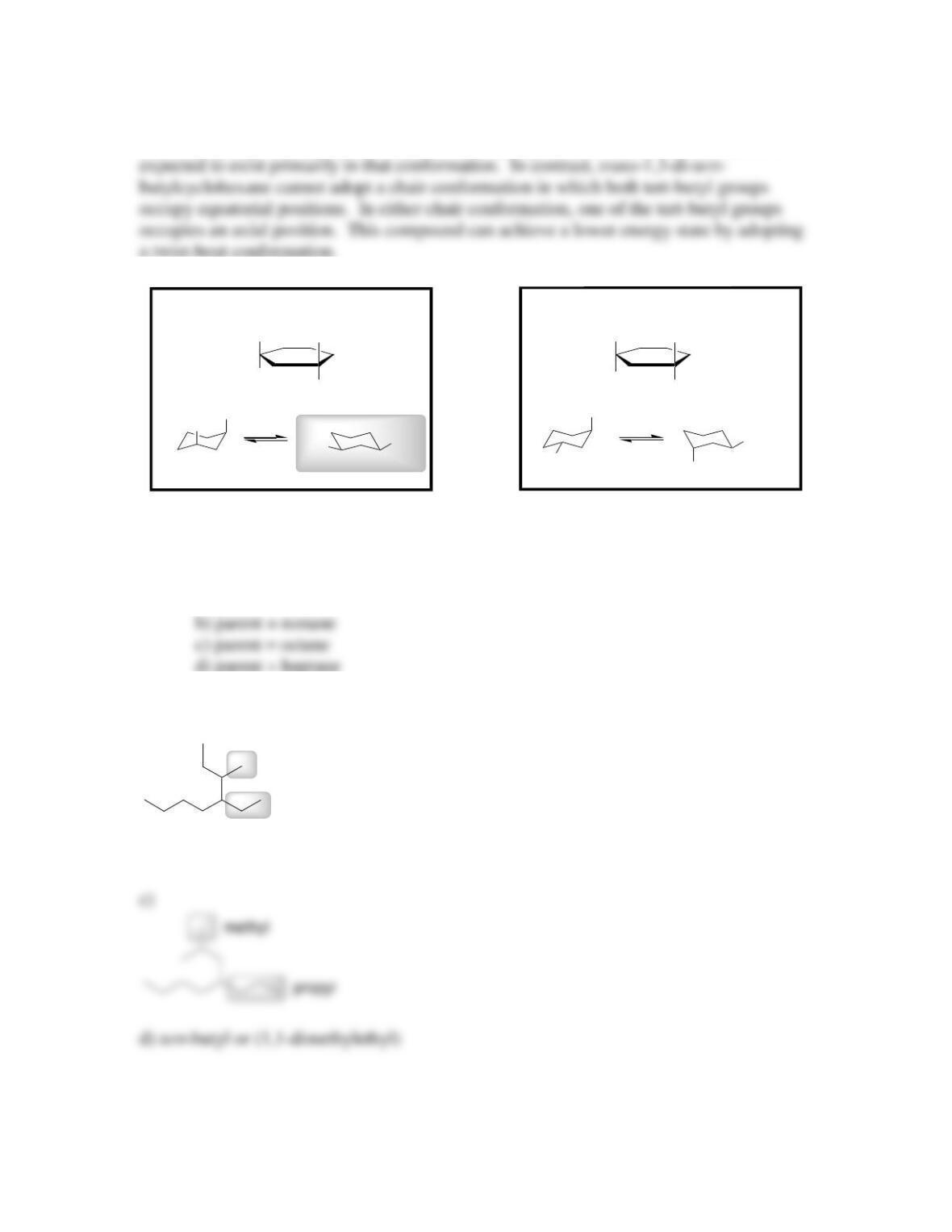
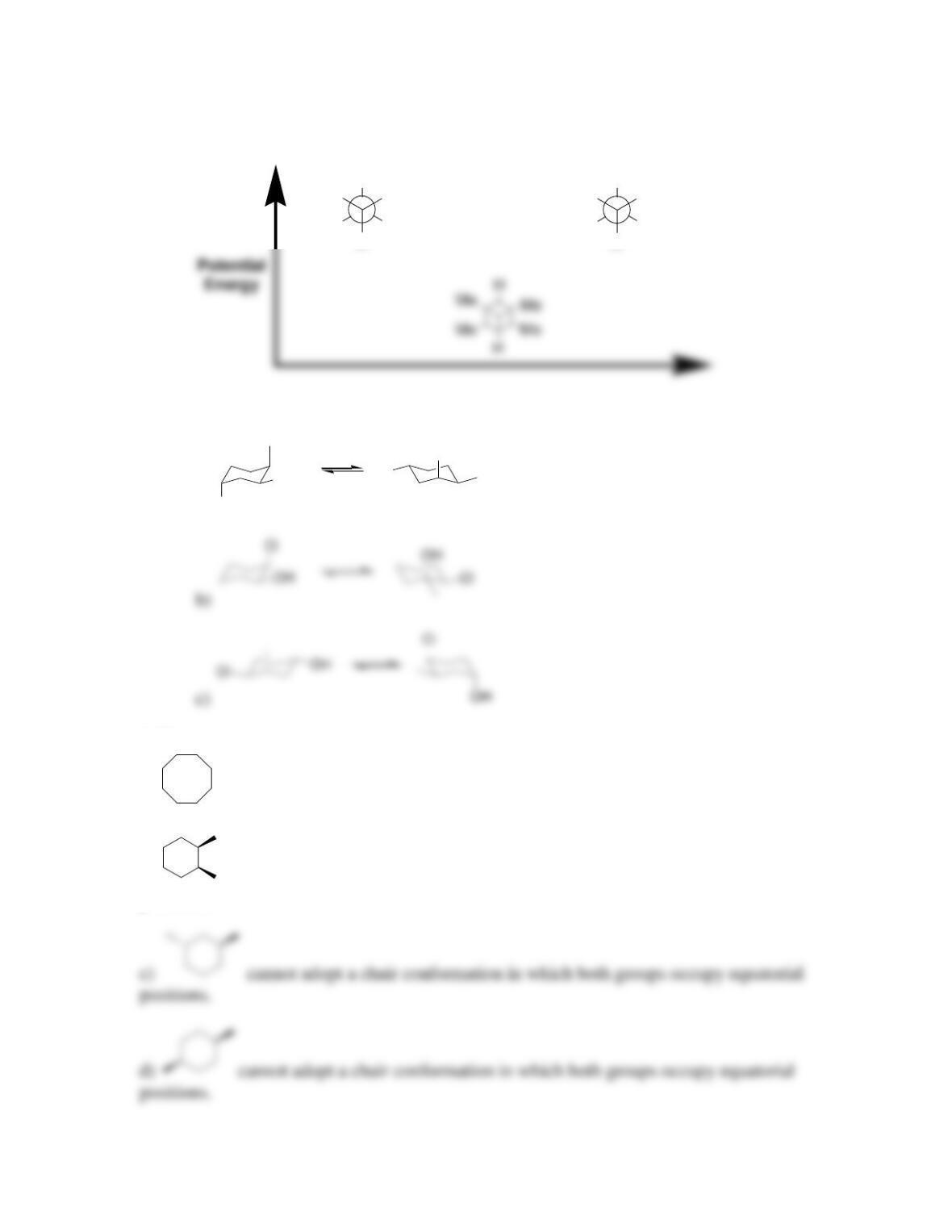
4.34. The two chair conformations of lindane are degenerate. There is no difference in
energy between them.
4.35. trans-1,4-di-tert-butylcyclohexane exists predominantly in a chair conformation,
because both substituents can occupy equatorial positions. In contrast, cis-1,4-di-tert-
4.36. cis-1,3-dimethylcyclohexane is expected to be more stable than trans-1,3-
dimethylcyclohexane because the former can adopt a chair conformation in which both
substituents are in equatorial positions (highlighted below):
CH
3
CH
3
CH
3
H
H
CH
cis-1,3-dimethylcyclohexane trans-1,3-dimethylcyclohexane
4.37. trans-1,4-dimethylcyclohexane is expected to be more stable than cis-1,4-
dimethylcyclohexane because the latter can adopt a chair conformation in which both
substituents are in equatorial positions (highlighted below):
cis-1,4-dimethylcyclohexane trans-1,4-dimethylcyclohexane