714
CHAPTER 27
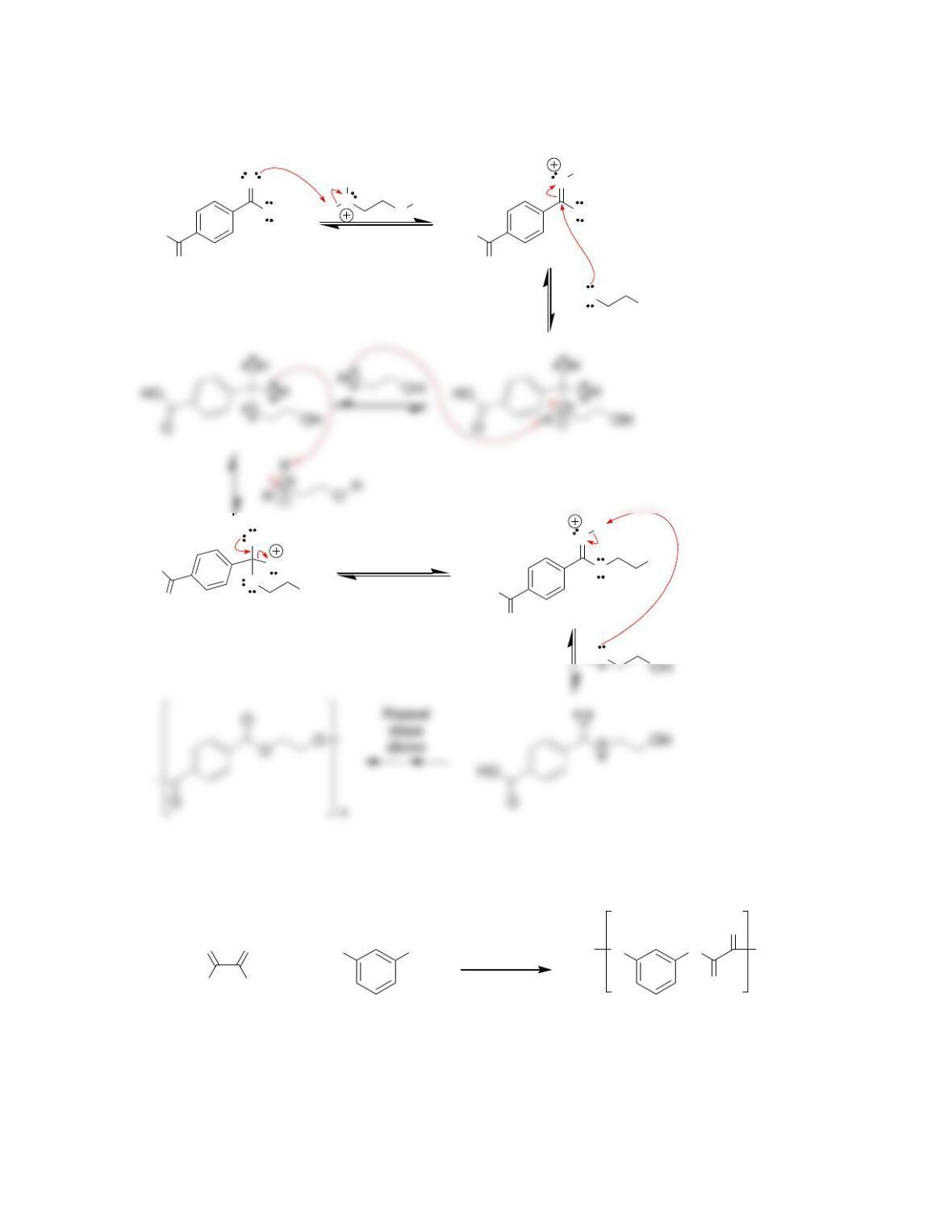
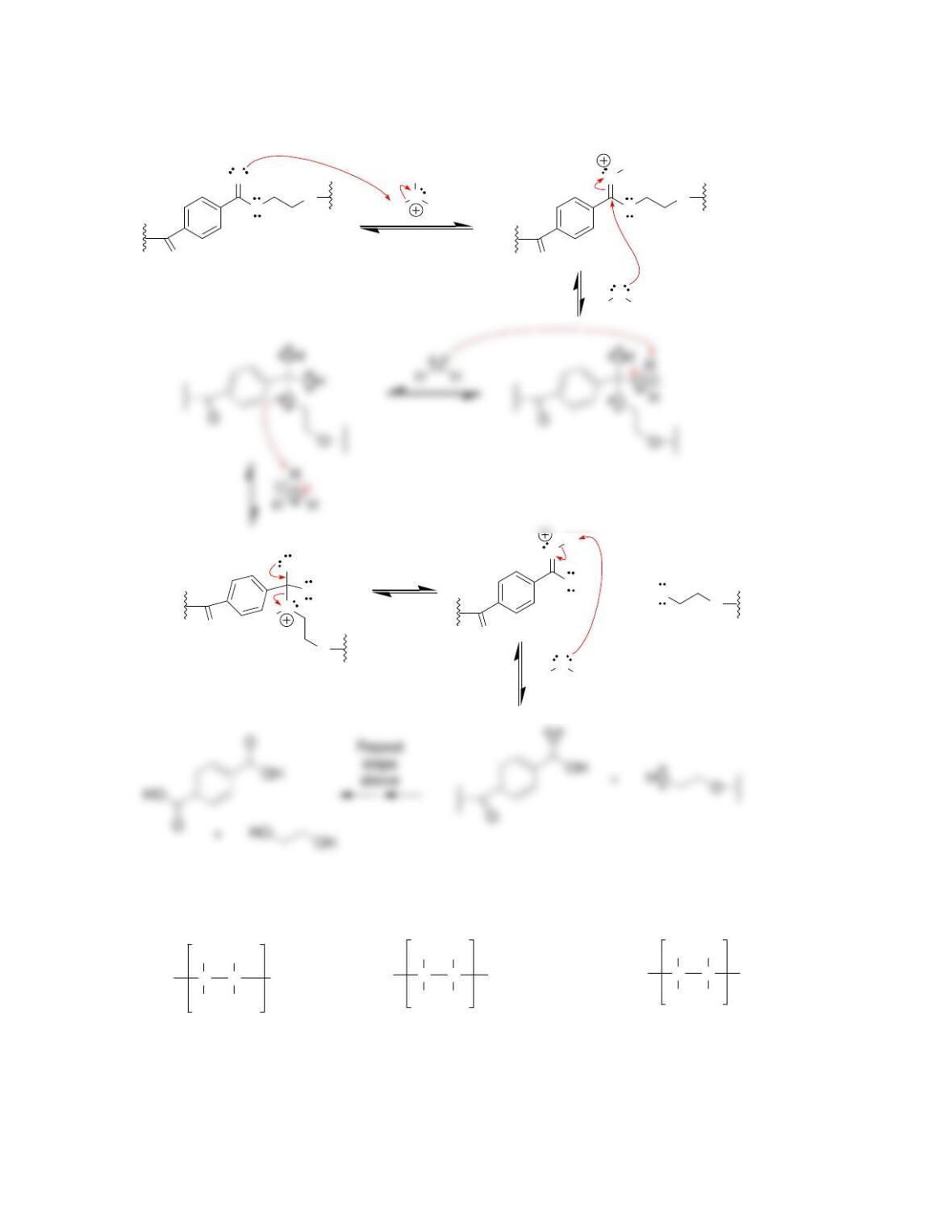
27.34. Nitro groups are among the most powerful electron-withdrawing groups, and a
27.35. Shower curtains are made from PVC, which is a thermoplastic polymer. To
prevent the polymer from being brittle, the polymer is prepared in the presence of
27.36. Polyformaldehyde, sold under the trade name Delrin, is a strong polymer used in
the manufacture of many guitar picks. It is prepared via the acid-catalyzed
polymerization of formaldehyde. [[LO 27.4]] [[LO 27.5]]
27.37. It bears an electron-withdrawing group (CN) that can stabilize a negative charge
27.38. The nitro group serves as a reservoir of electron density that stabilizes an negative
charge via resonance (see Chapter 19).
27.39. The methoxy group is an electron donating group that stabilizes a positive charge
via resonance (see Chapter 19).
27.40. A methoxy group can only donate electron density via resonance if it is located in
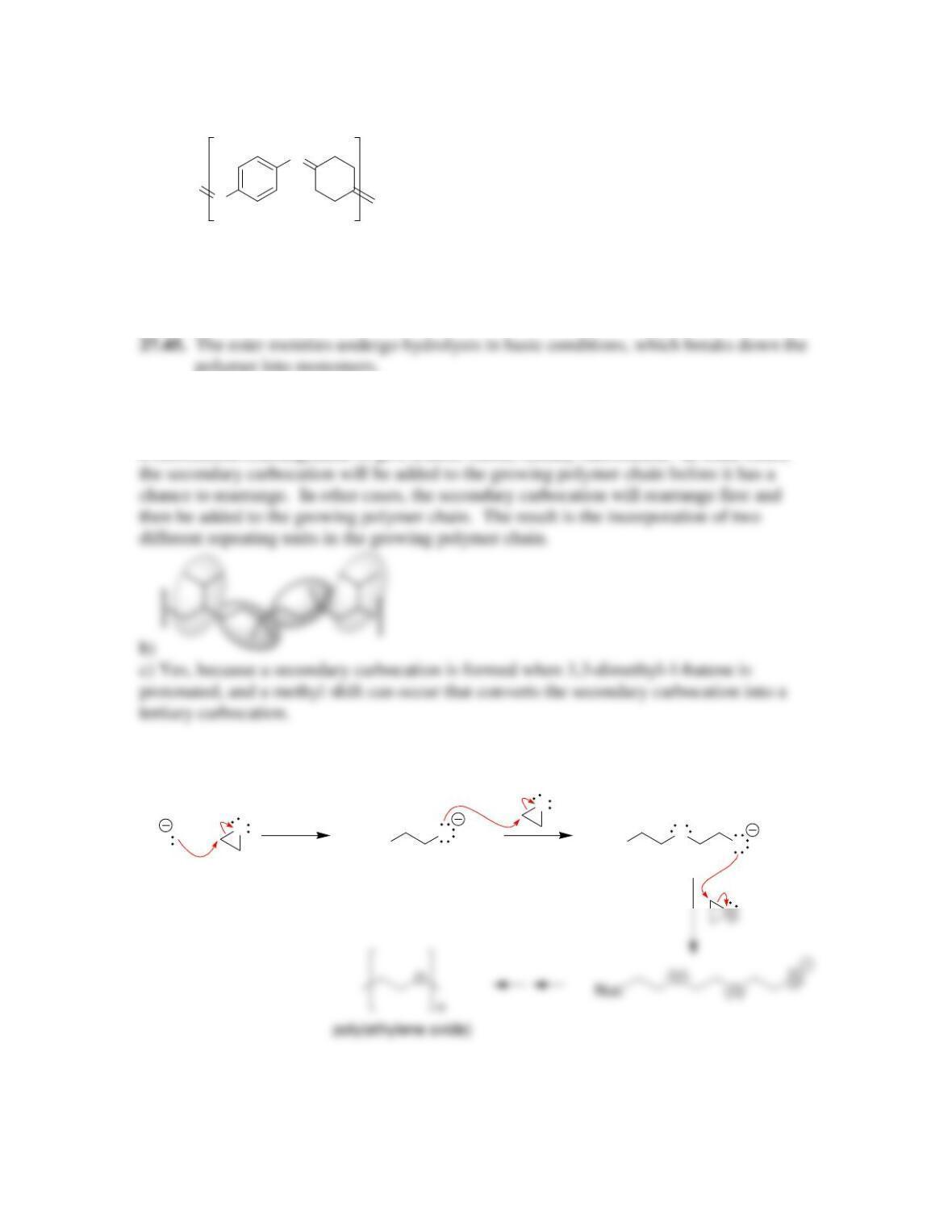
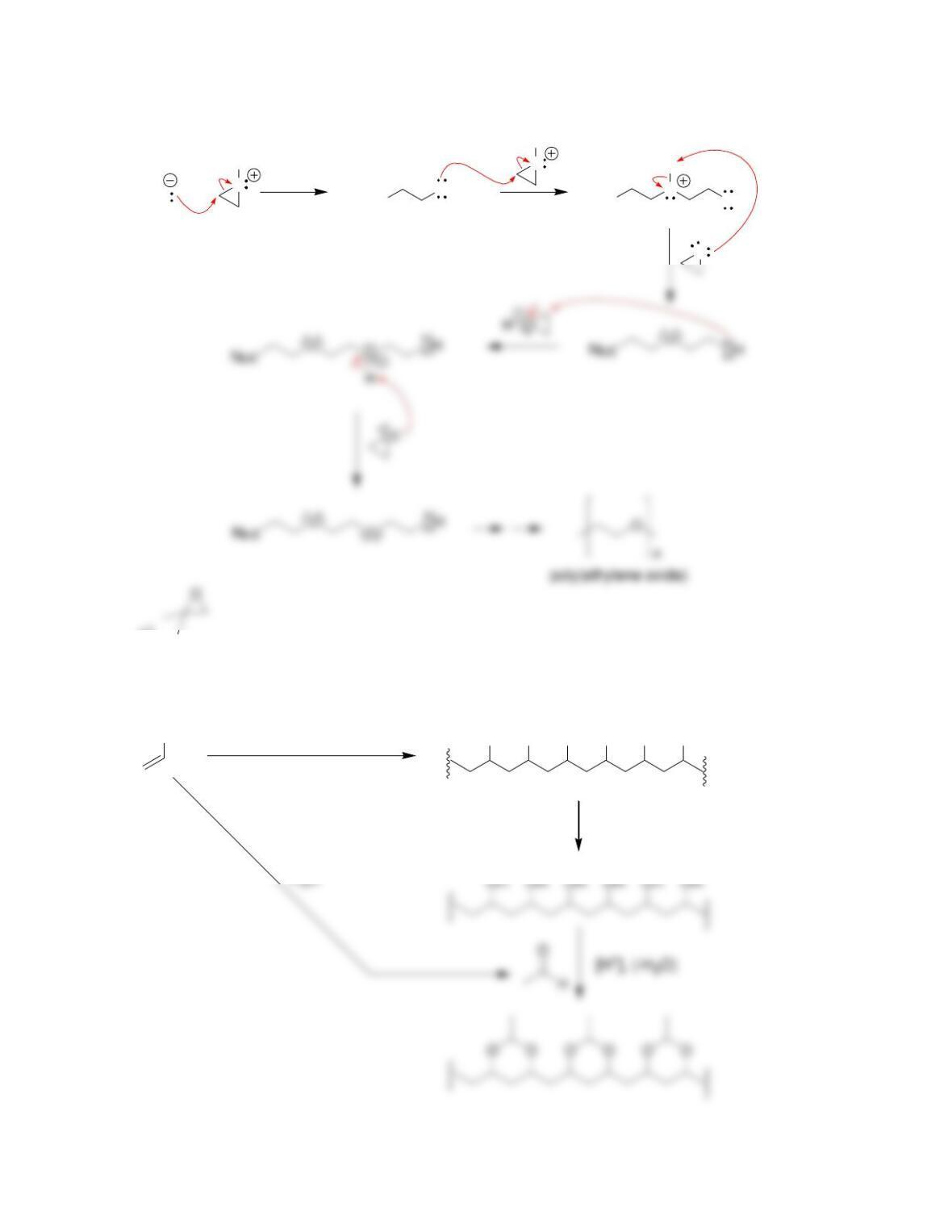
27.41.
27.42.
a)
OHHONN CC OO
+
b) Step growth c) Addition polymer