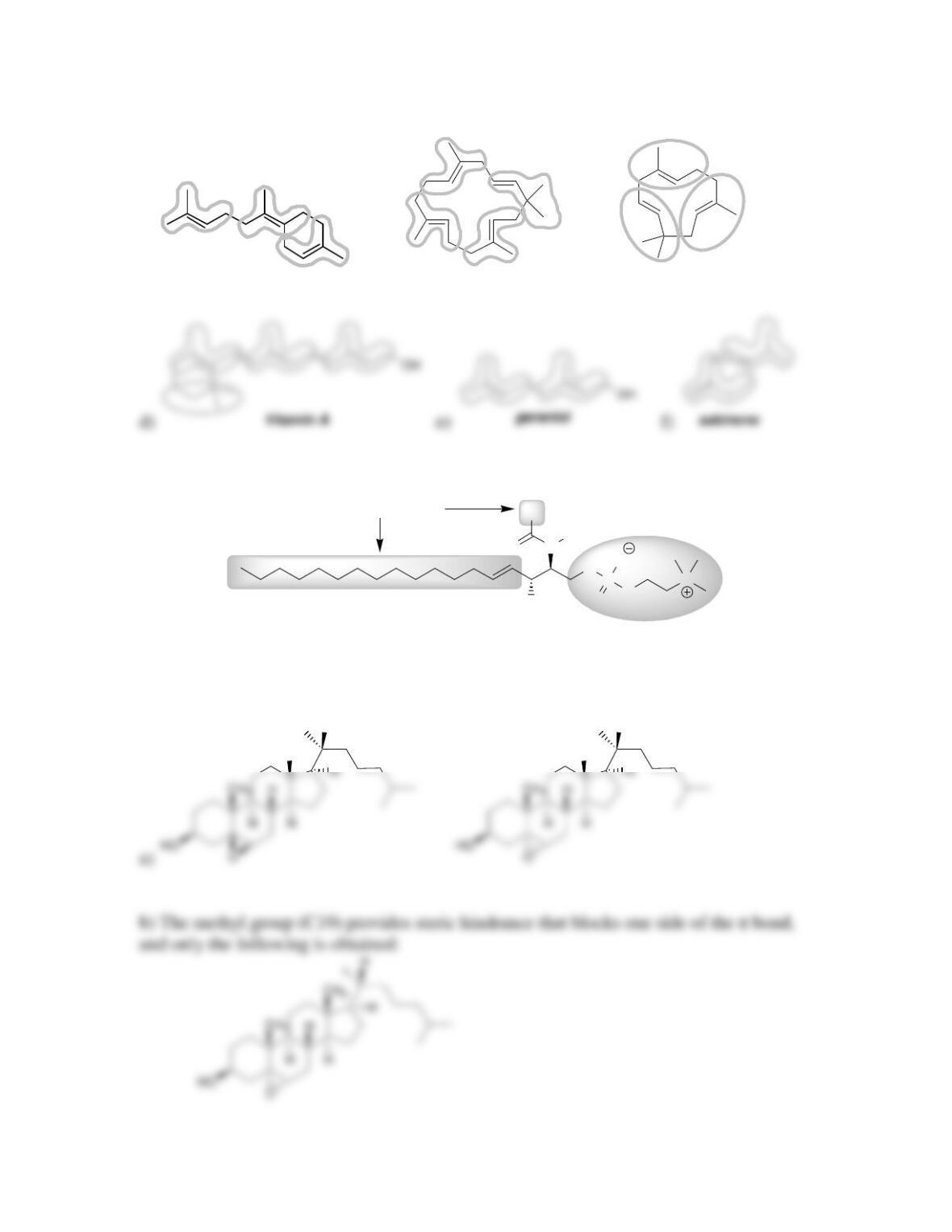
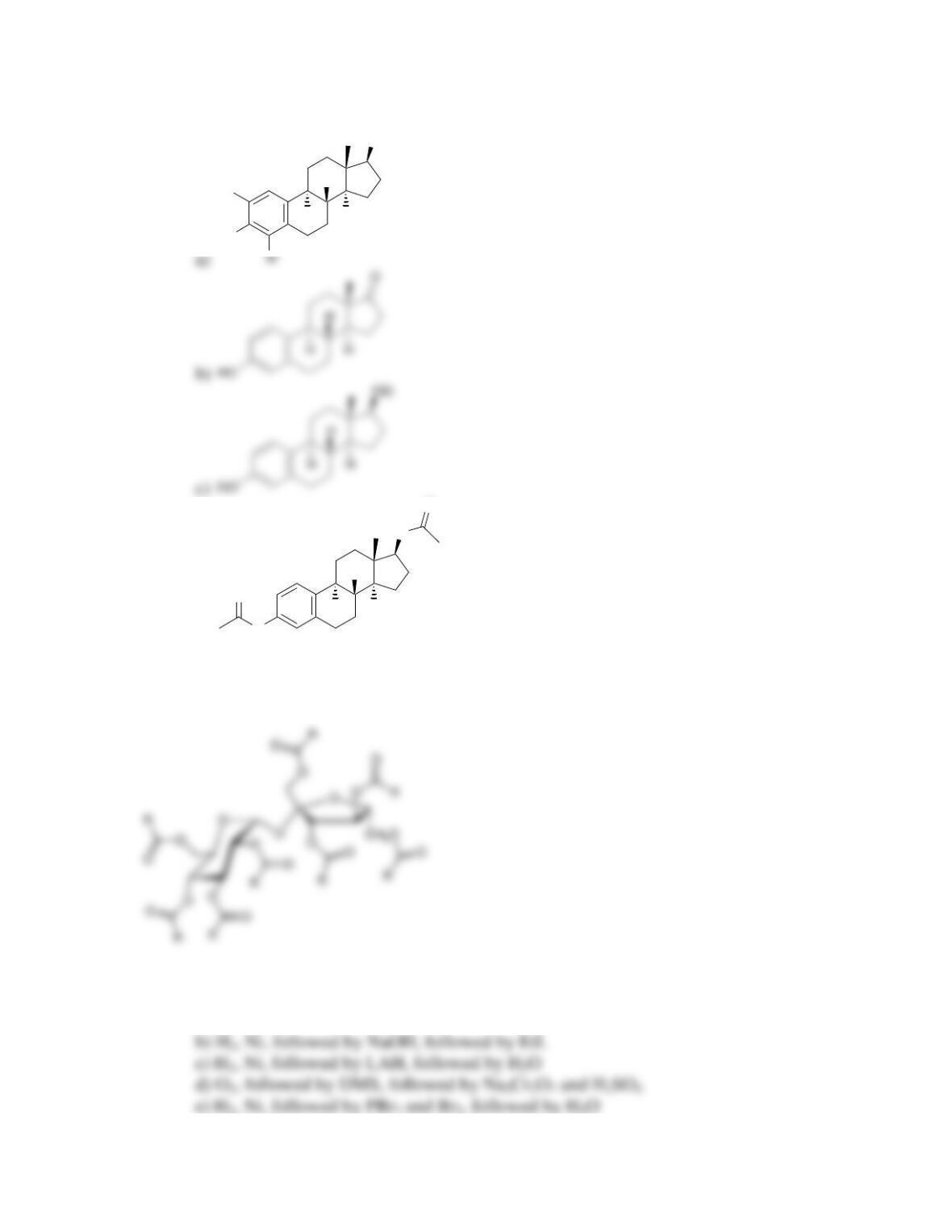
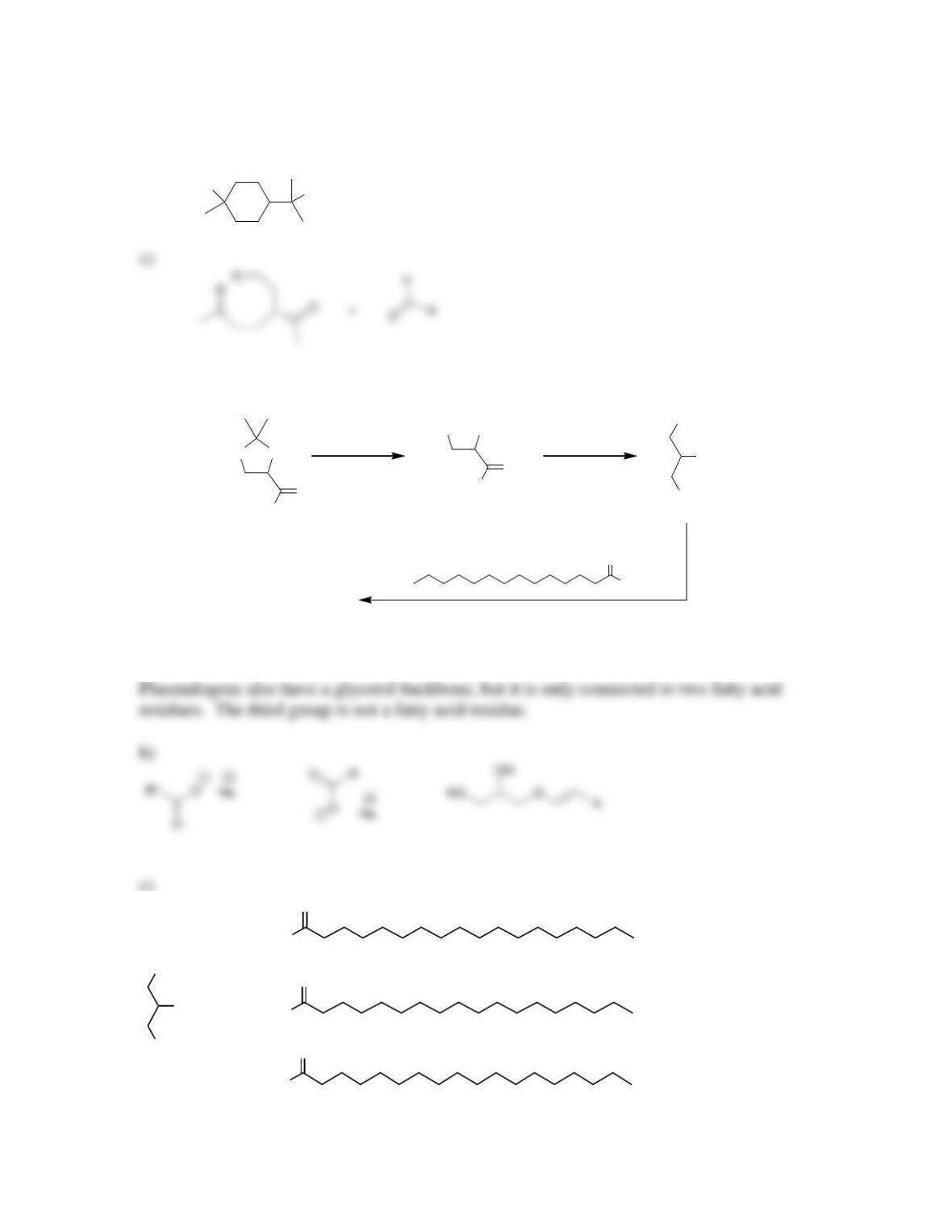
Chapter 26
Lipids
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 26. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
____________. The presence of a _____ double bond causes a decrease in the
melting point.
• Triglycerides that are solids at room temperature are called ______, while those
that are liquids at room temperature are called _______.
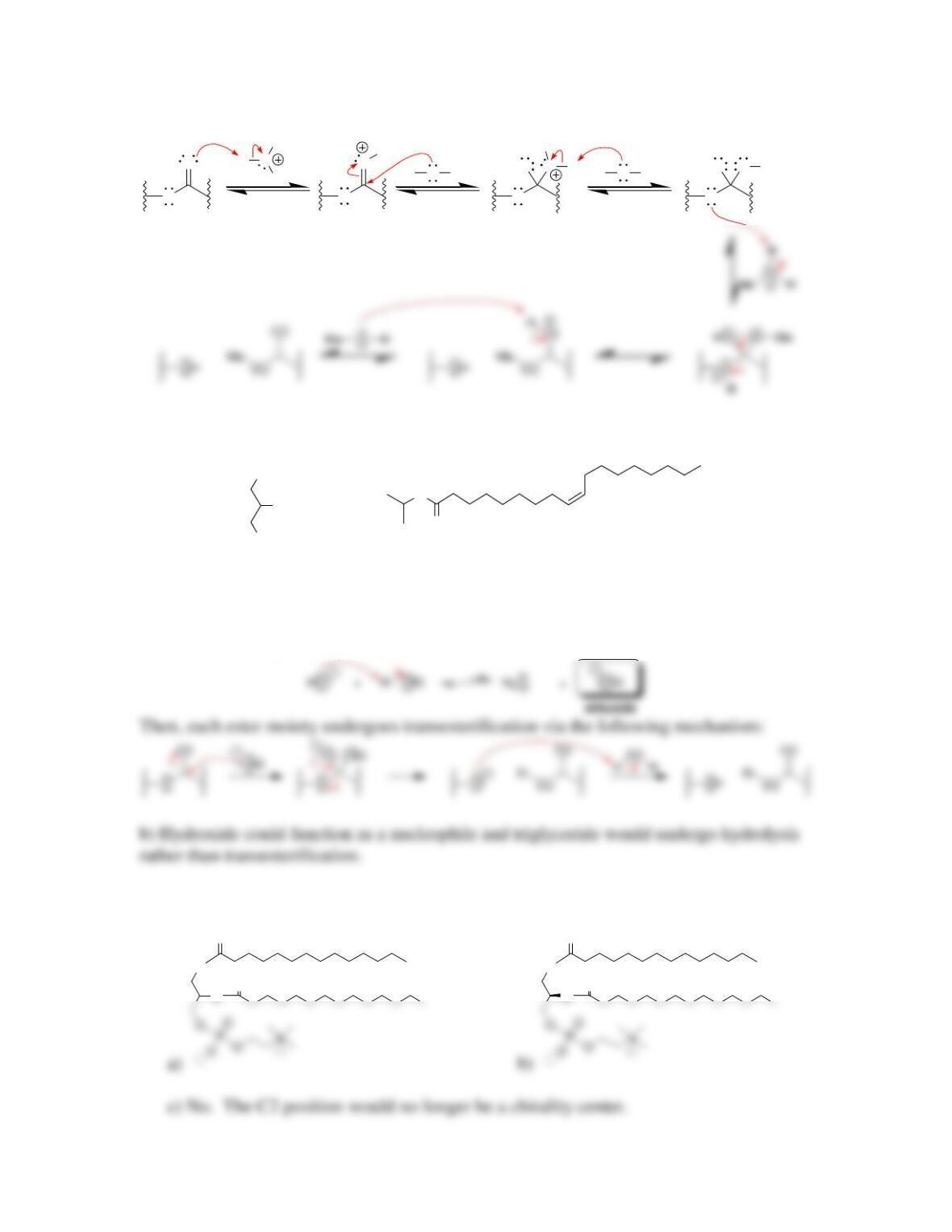
• Triglycerides containing unsaturated fatty acid residues will undergo
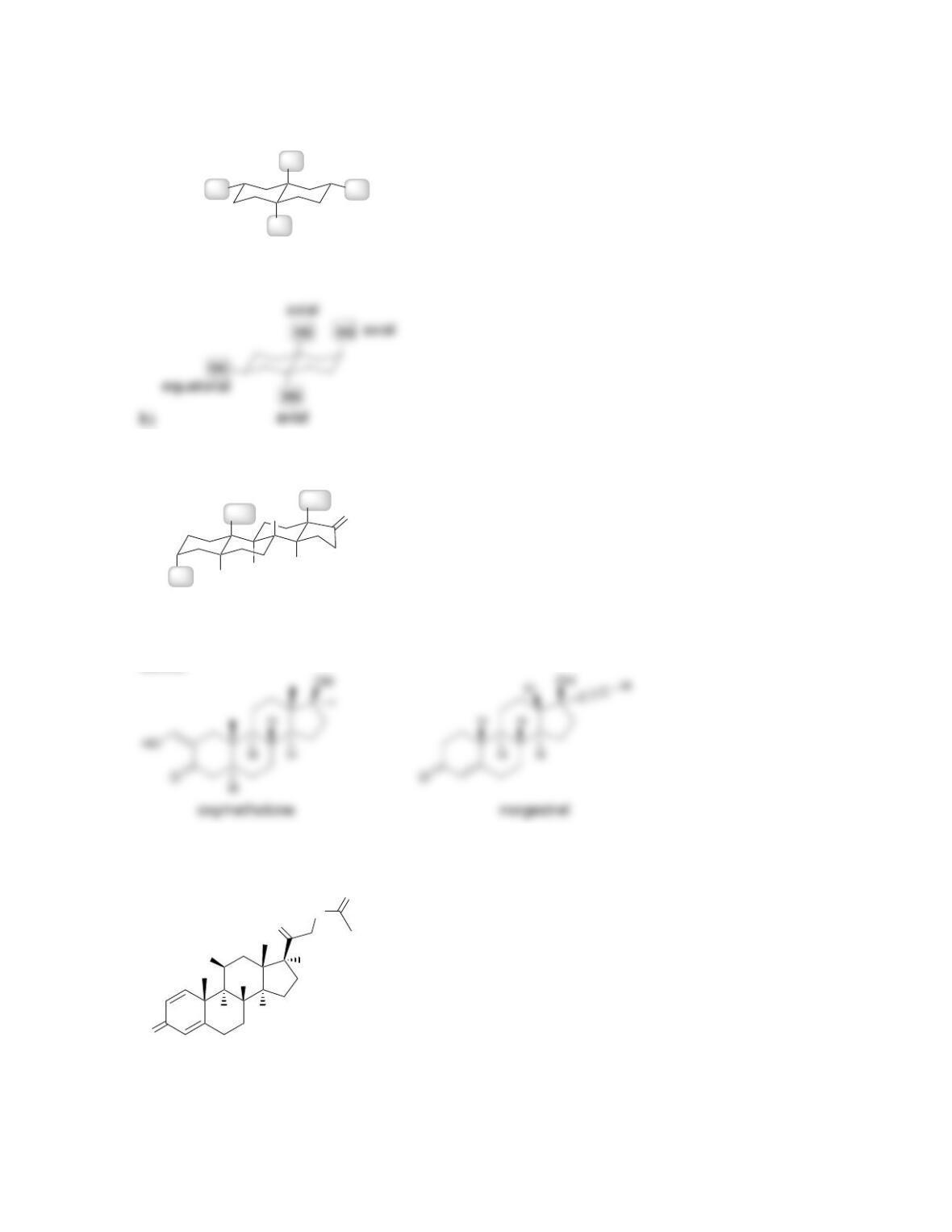
• The ring fusions are all _______ in most steroids, giving steroids their rigid
geometry.
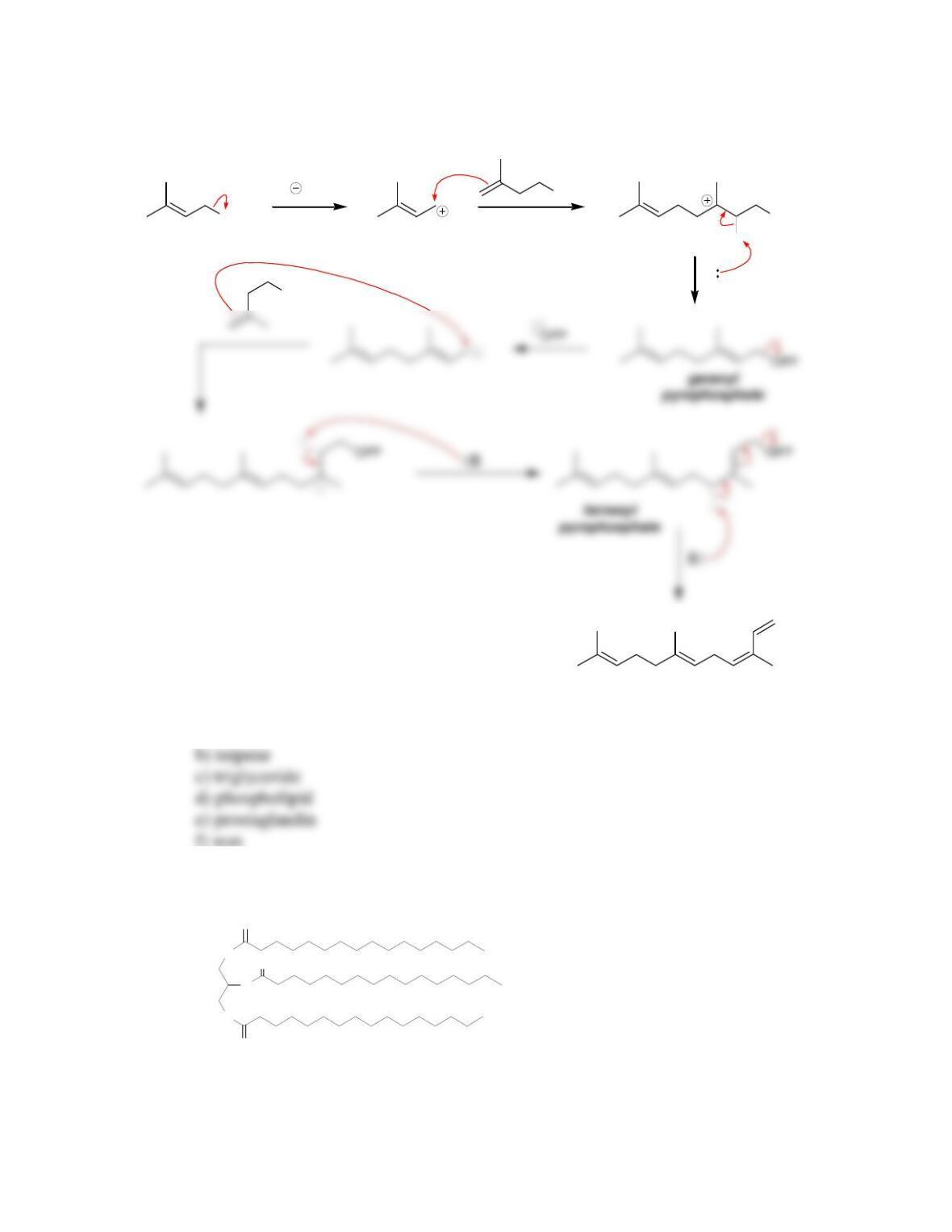
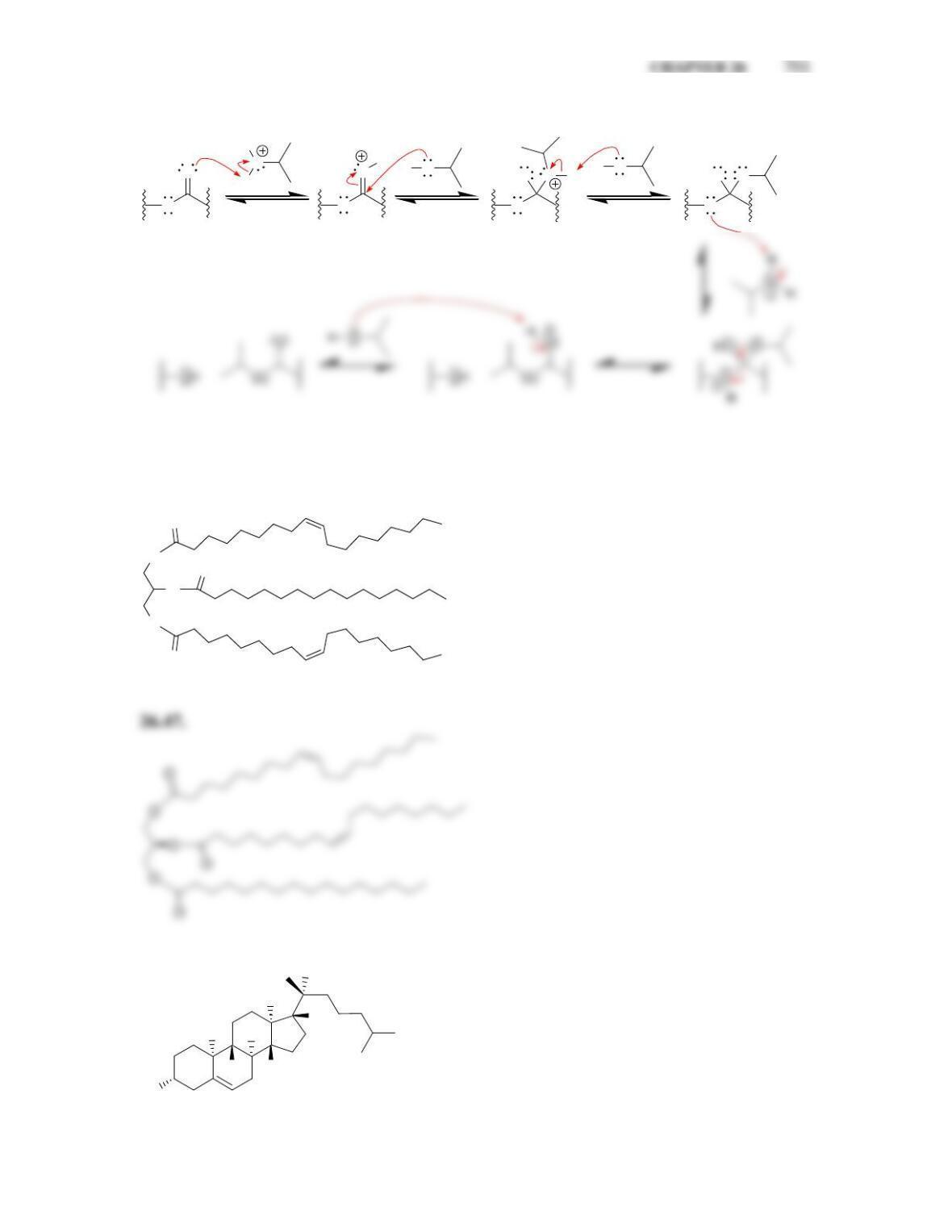
• All steroids, including cholesterol, are biosynthesized from ____________.
• Prostaglandins contain twenty carbon atoms and are characterized by a ______-
membered ring with two side chains.