Chapter 24
Carbohydrates
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 24. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
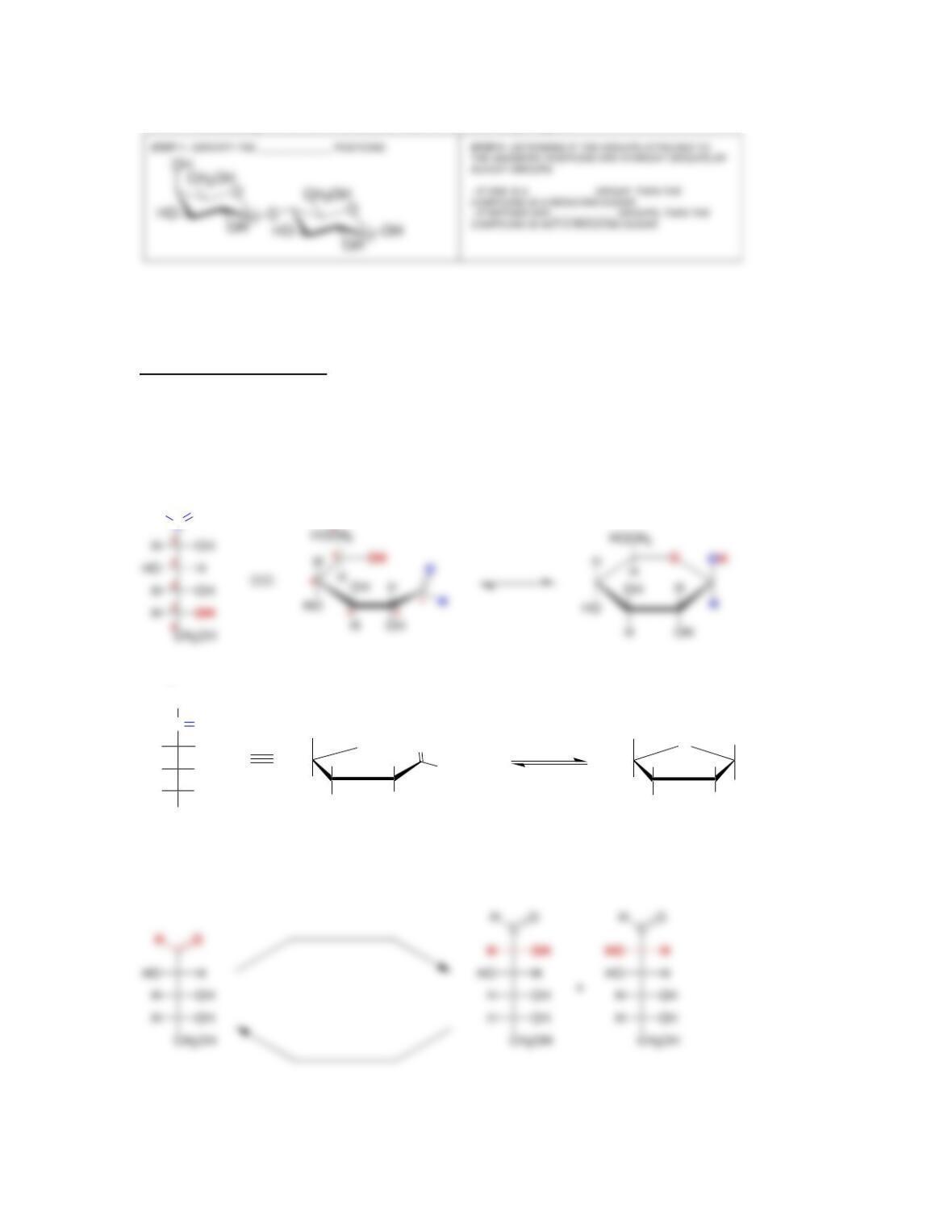
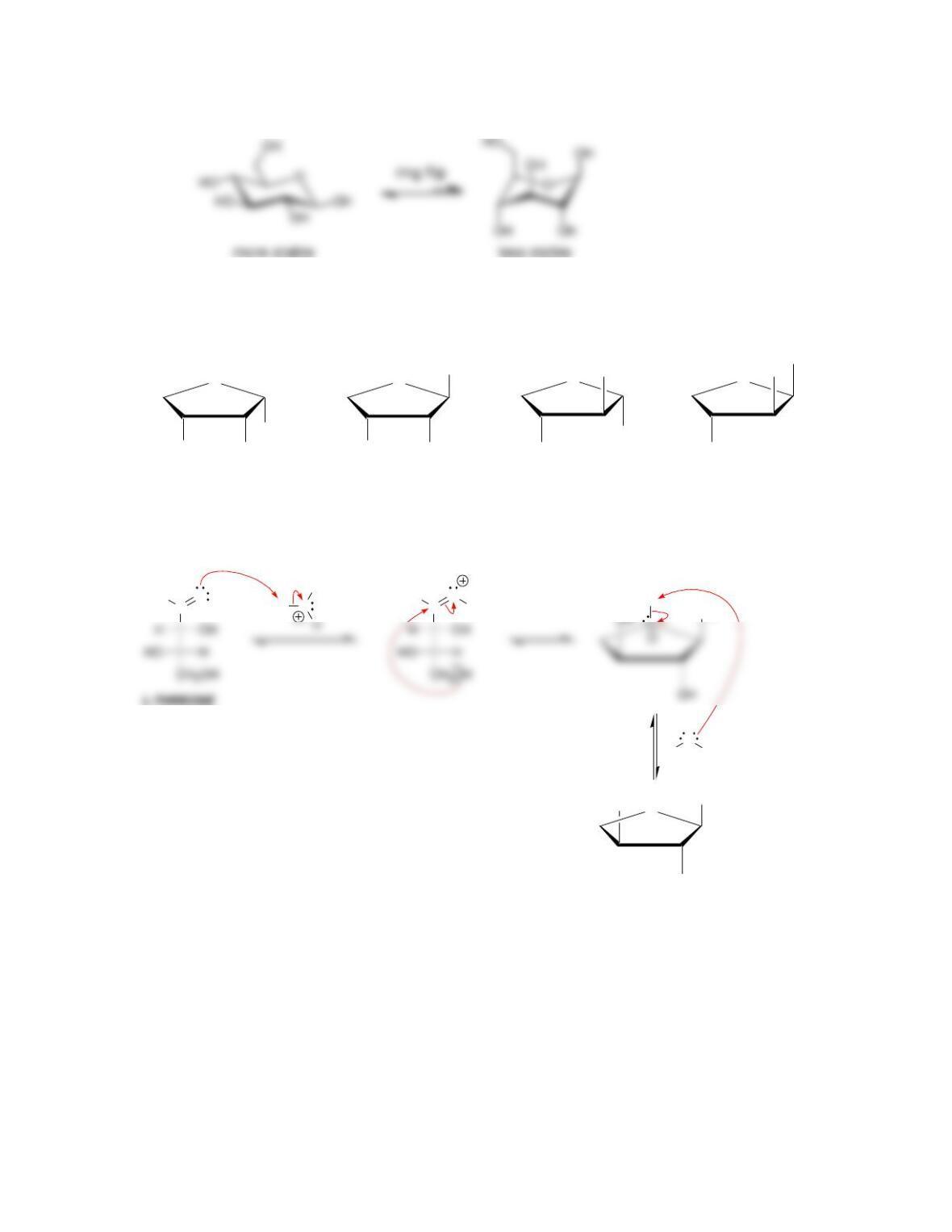
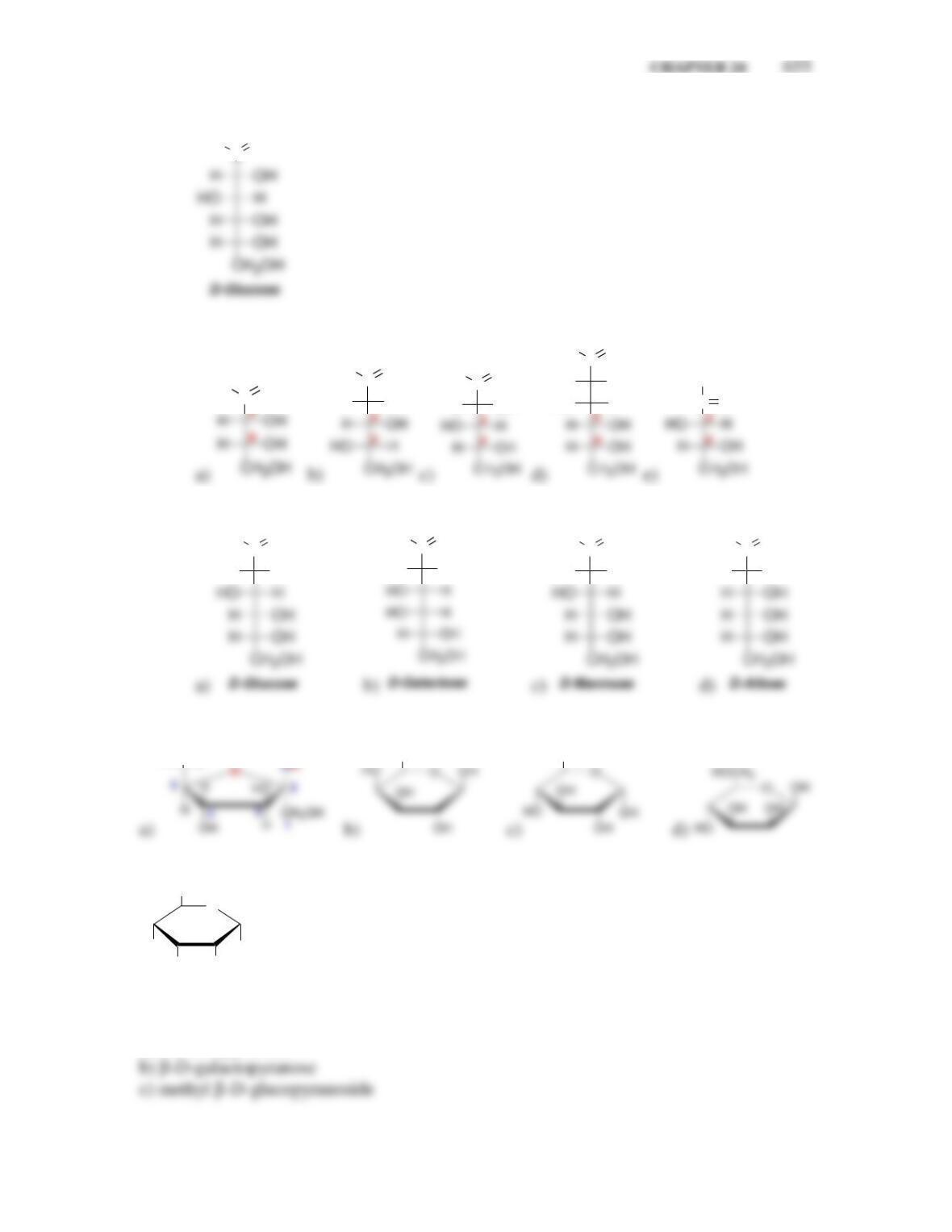
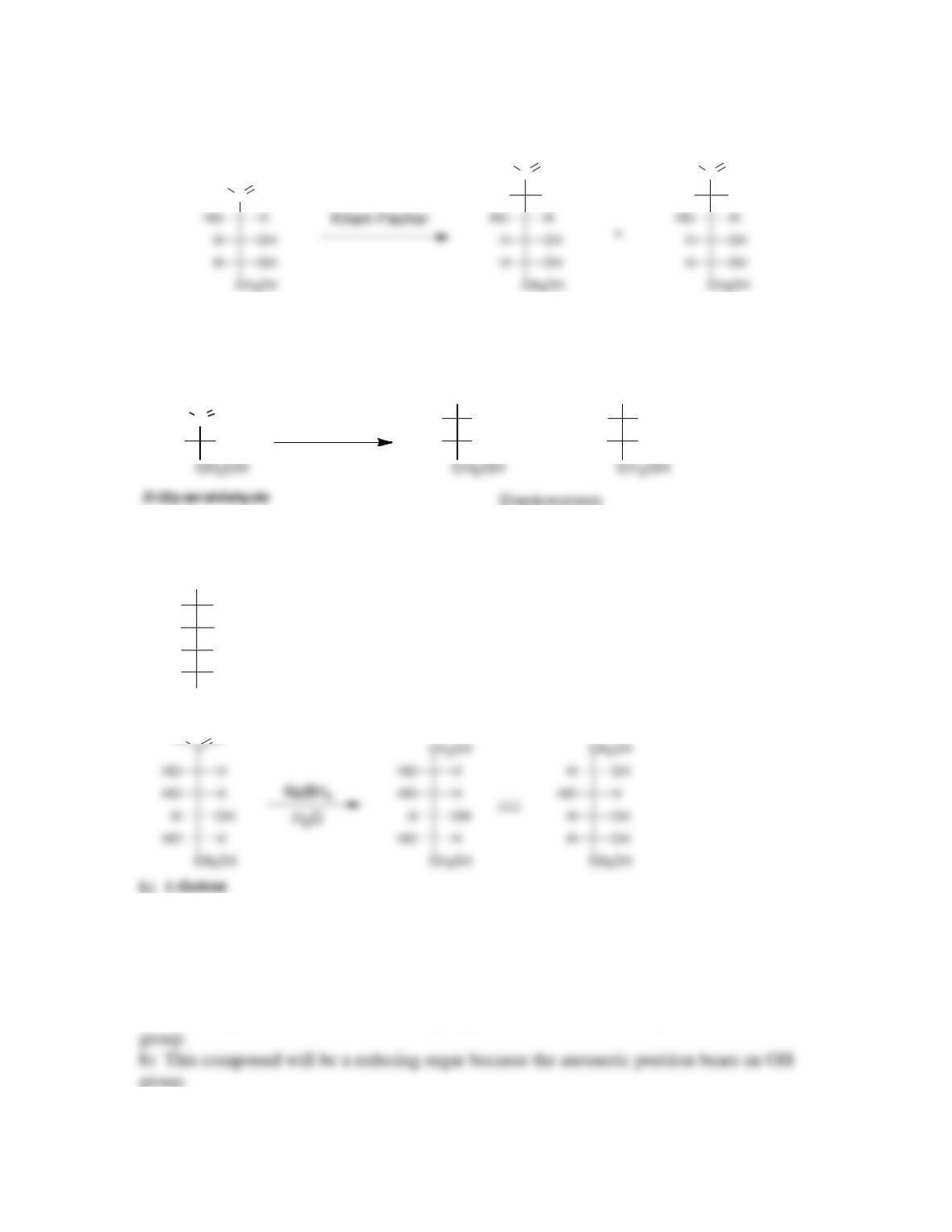
newly created chirality center is called the ___________ carbon.
• In the α anomer, the hydroxyl group at the anomeric position is ______ to the
CH
2
OH group, while in the β anomer, the hydroxyl group is ______ to the
CH
2
OH group.
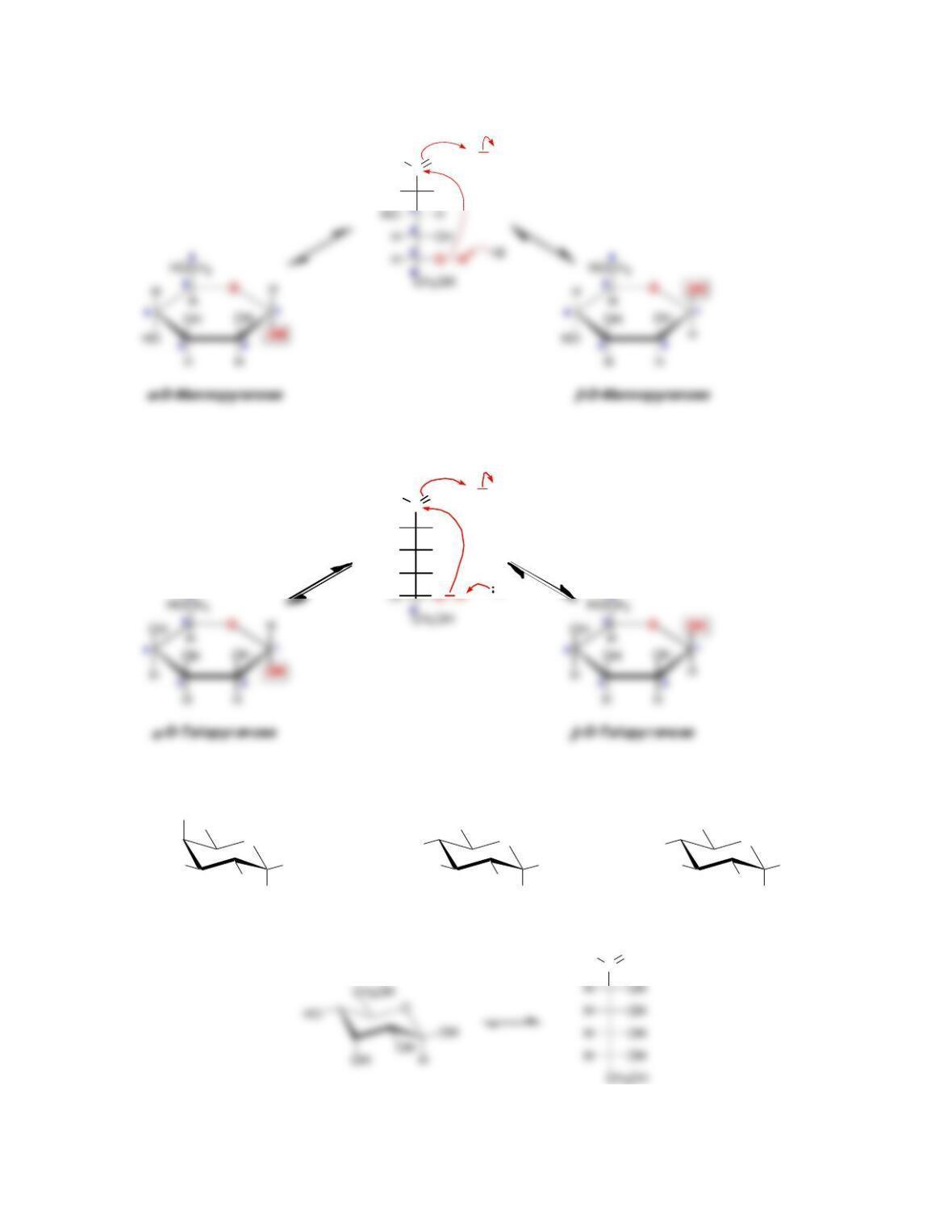
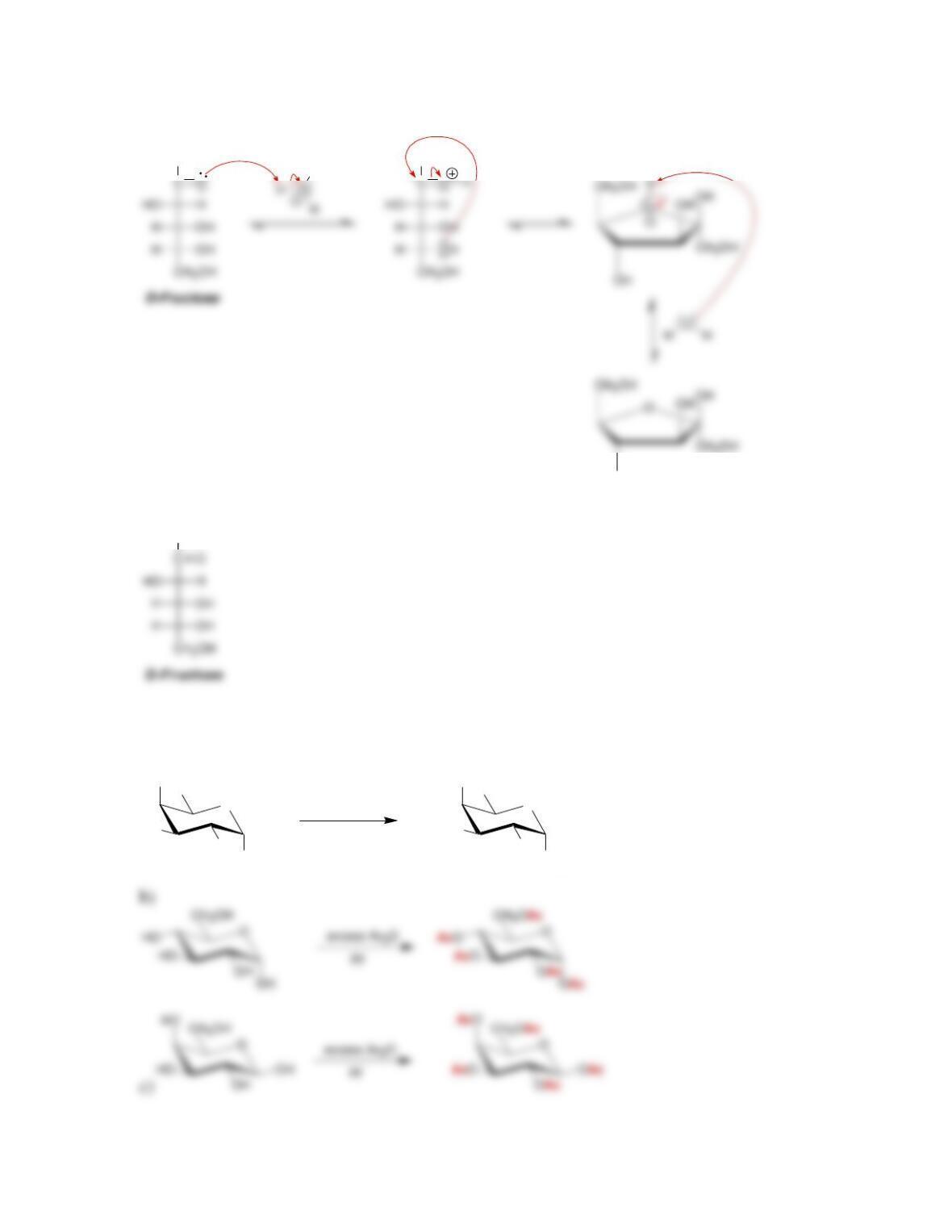
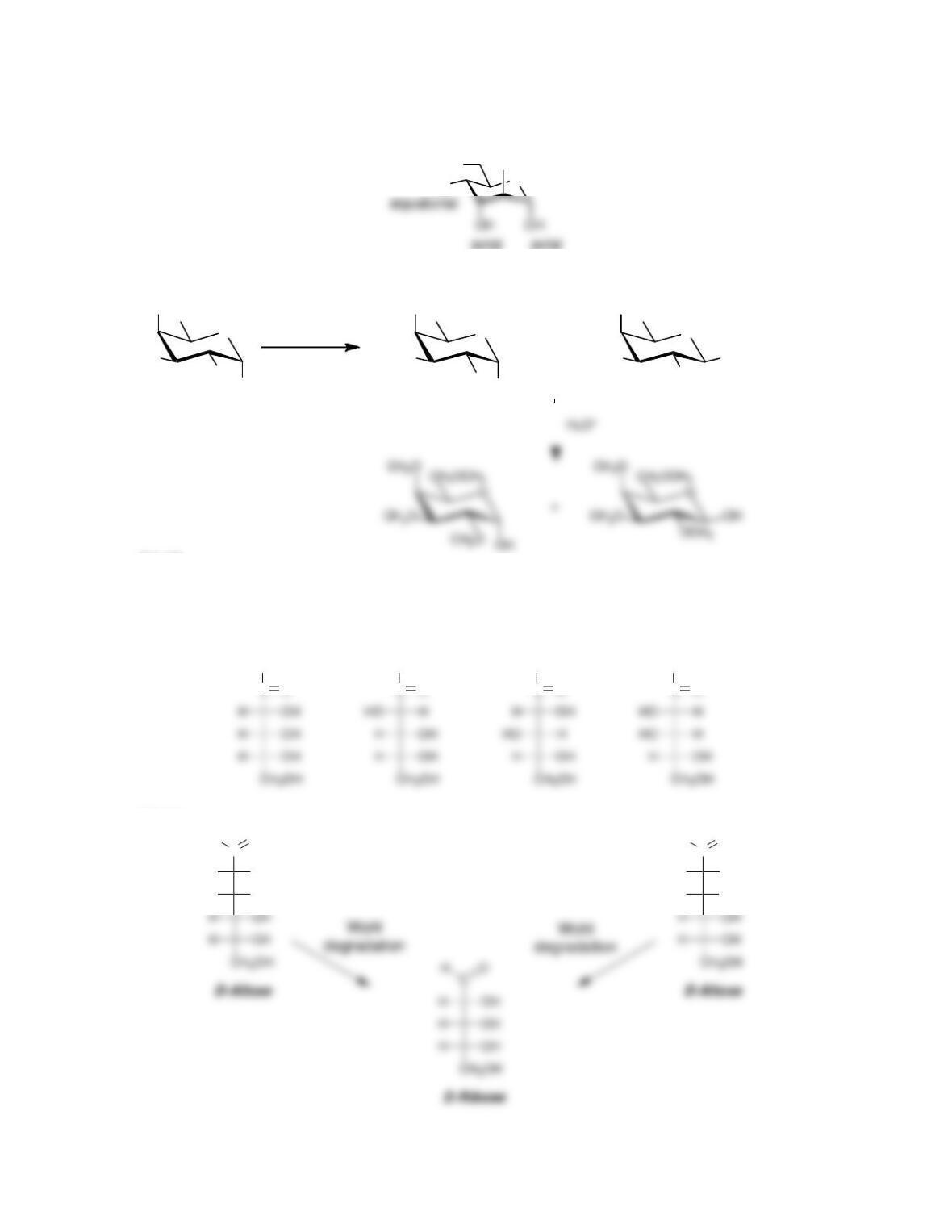
• Anomers equilibrate by a process called _____________, which is catalyzed by
yield an ___________.
• When treated with a suitable oxidizing agent, an aldose can be oxidized to yield
an __________.
• When treated with HNO
3
, an aldose is oxidized to give a dicarboxylic acid called
monosaccharides are converted into their corresponding N-glycosides.