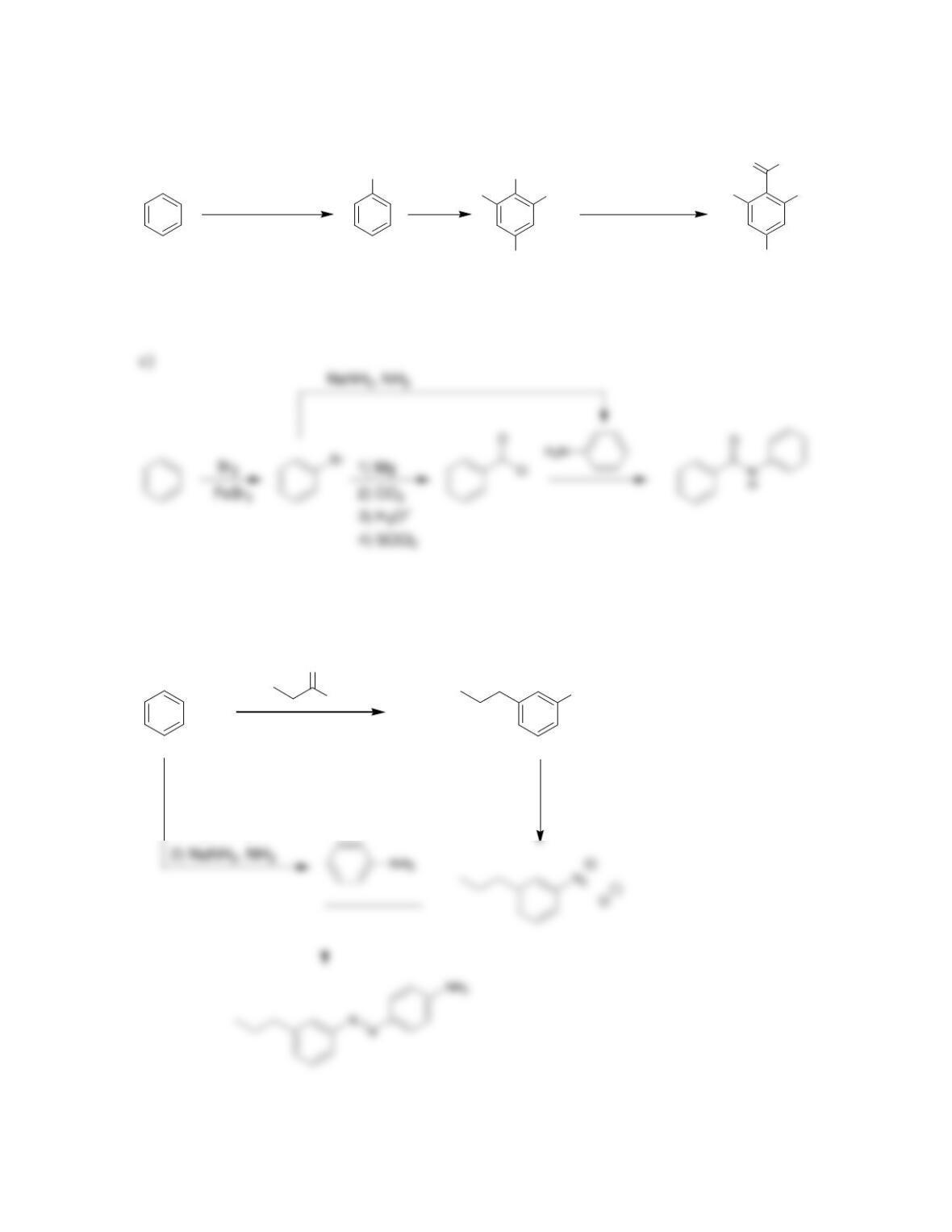
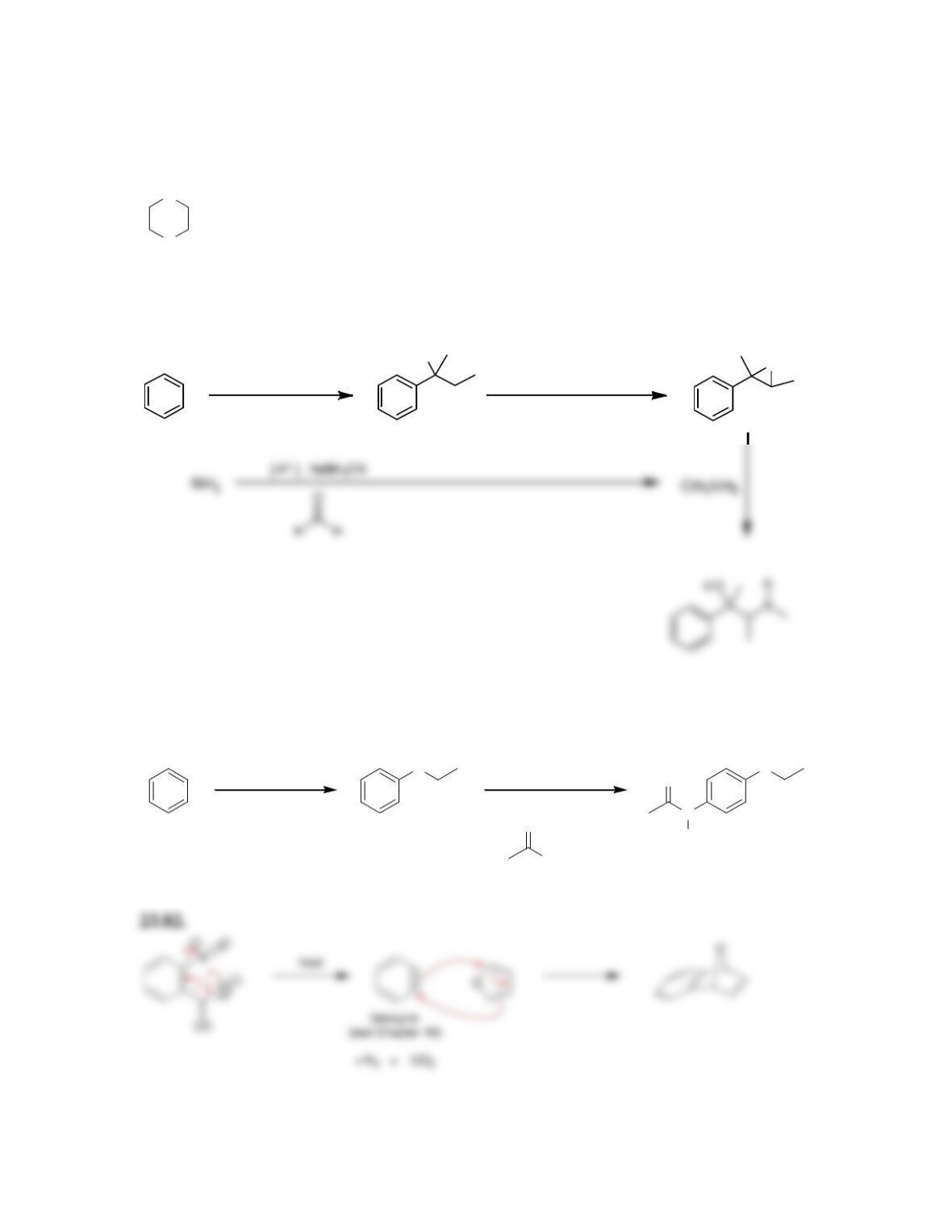
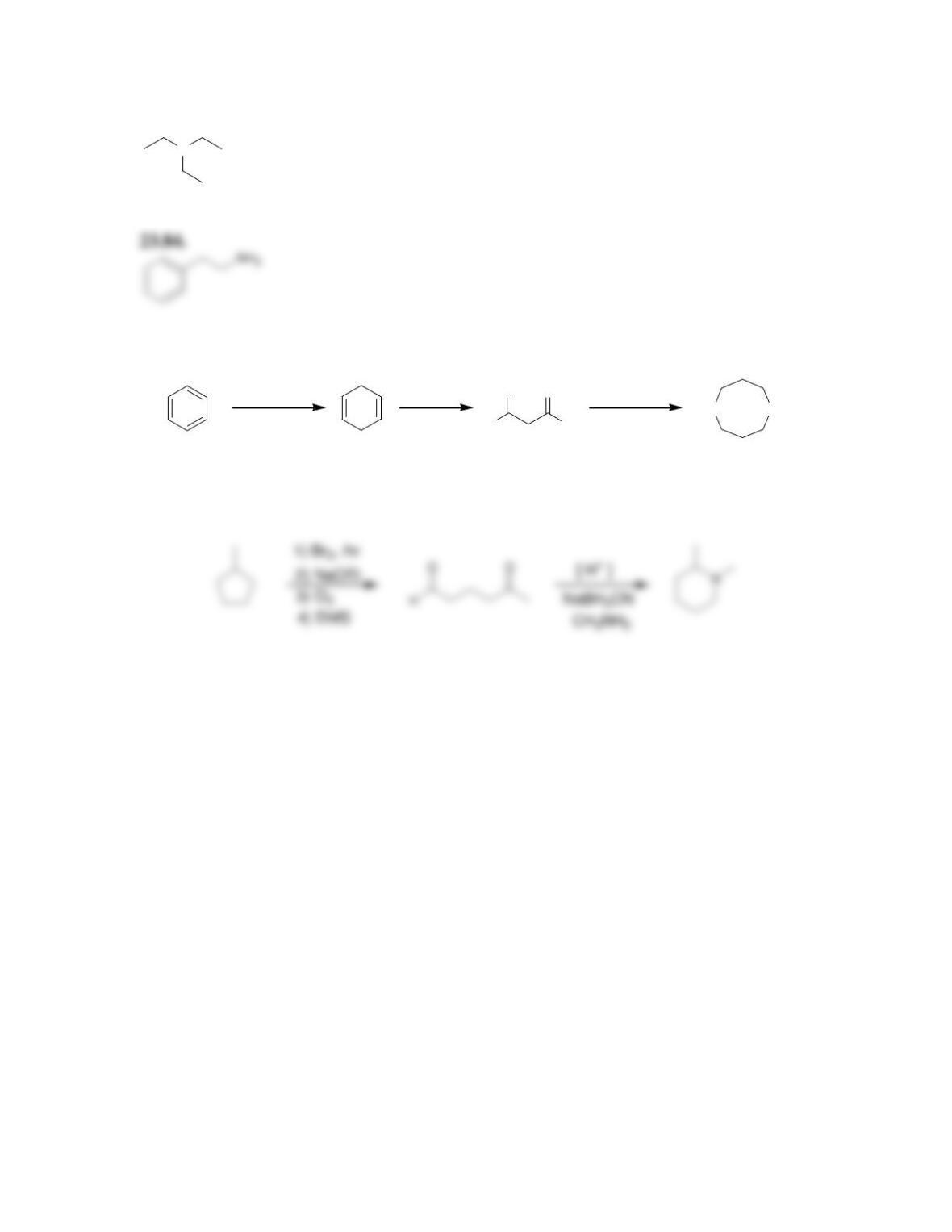
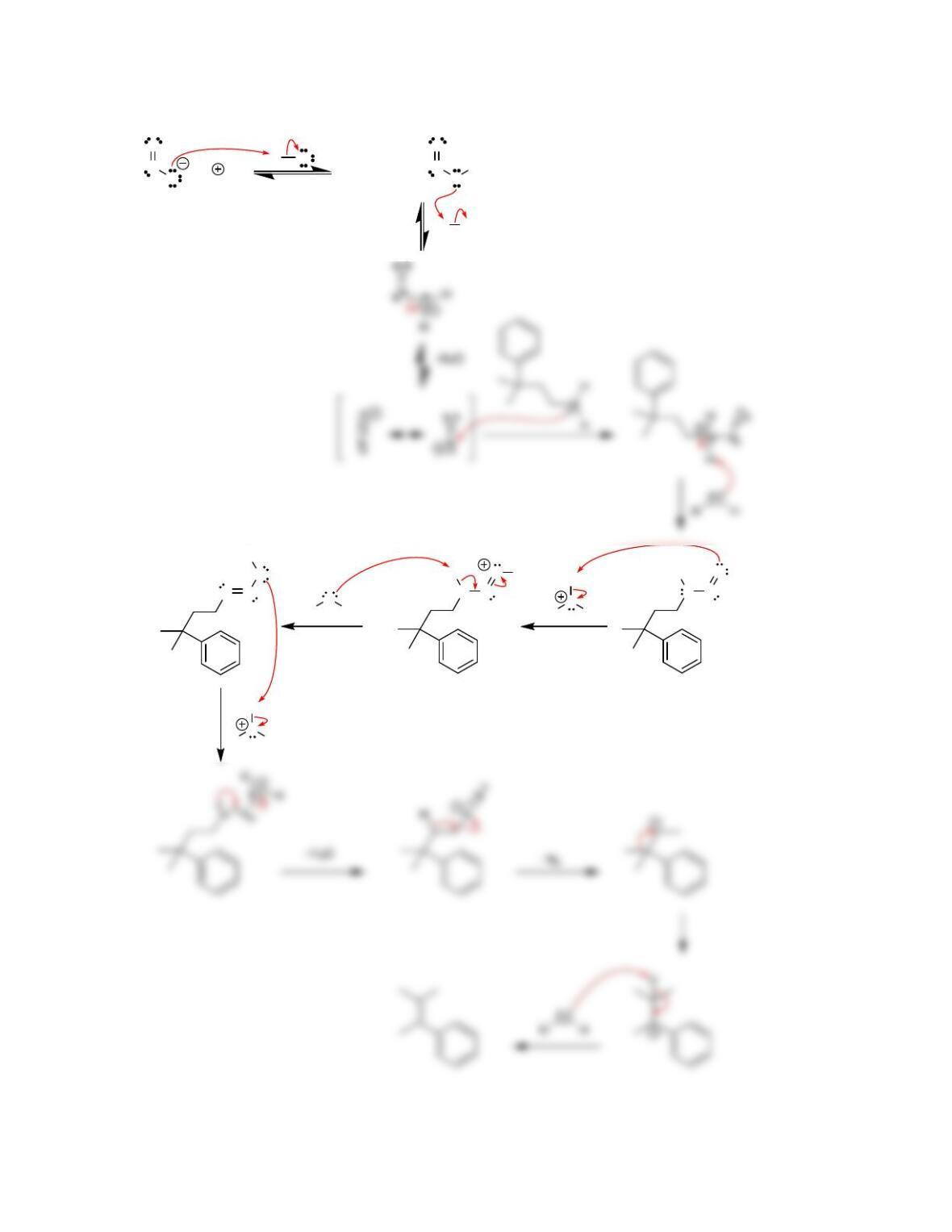
Chapter 23
Amines
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 23. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
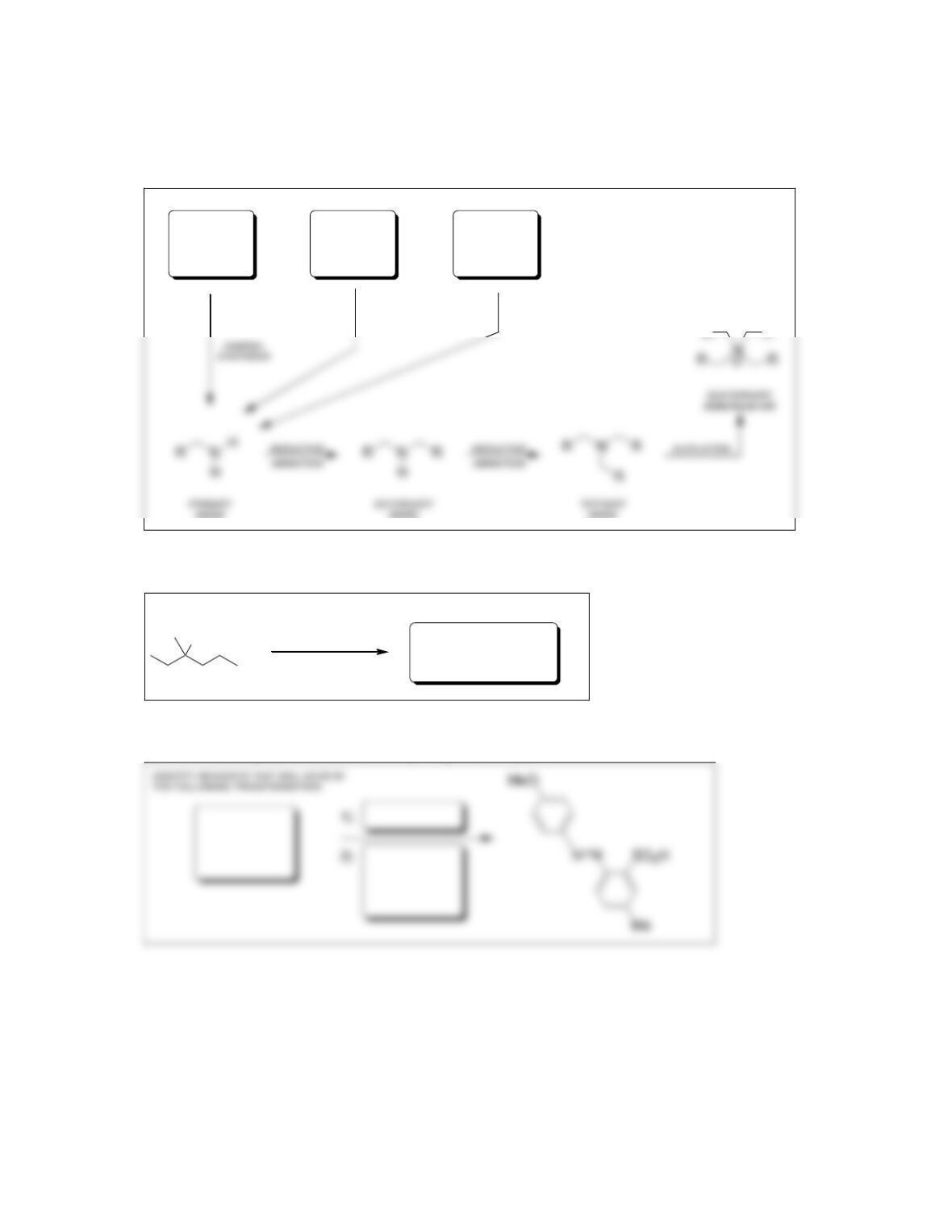
• Pyridine is a stronger base than pyrrole, because the lone pair in pyrrole
participates in ____________.
• An amine moiety exists primarily as ______________________________ at
physiological pH.
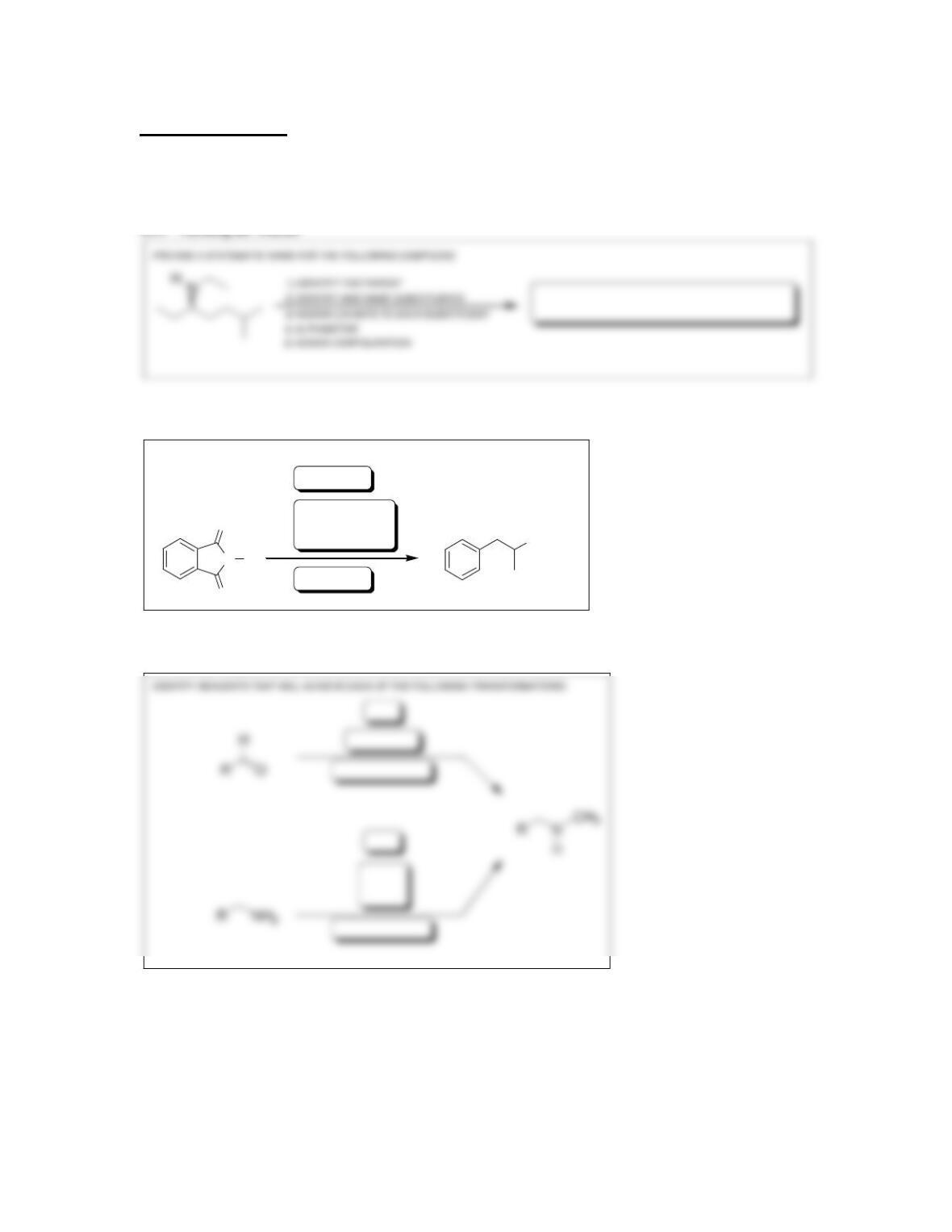
• The azide synthesis involves treating an ________________ with sodium azide,
followed by _______________.
• The __________ synthesis generates primary amines upon treatment of
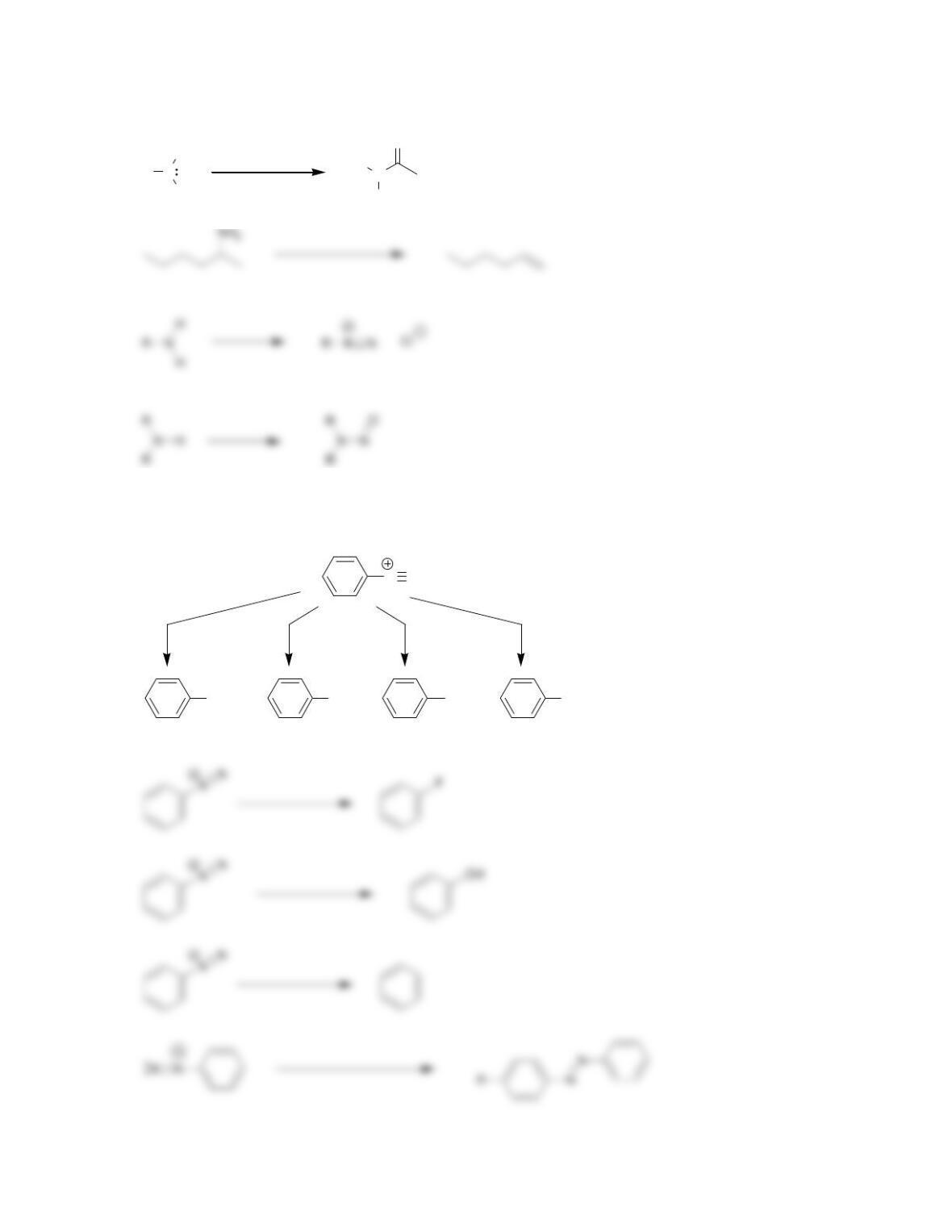
• Primary amines react with a nitrosonium ion to yield a ______________ salt in a
process called diazotization.
• Sandmeyer reactions utilize copper salts (CuX), enabling the installation of a
halogen or a ________ group.
• In the Schiemann reaction, an aryl diazonium salt is converted into a