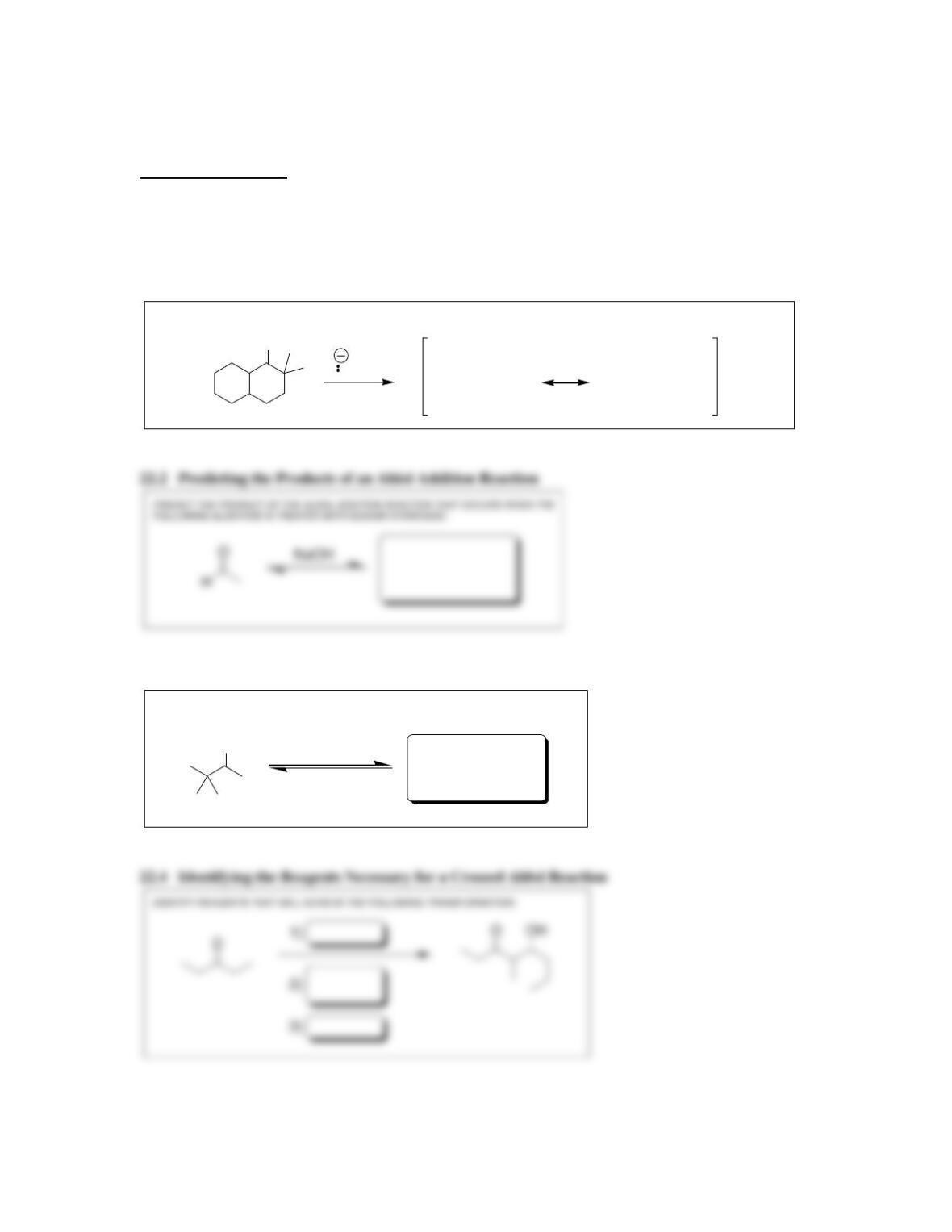
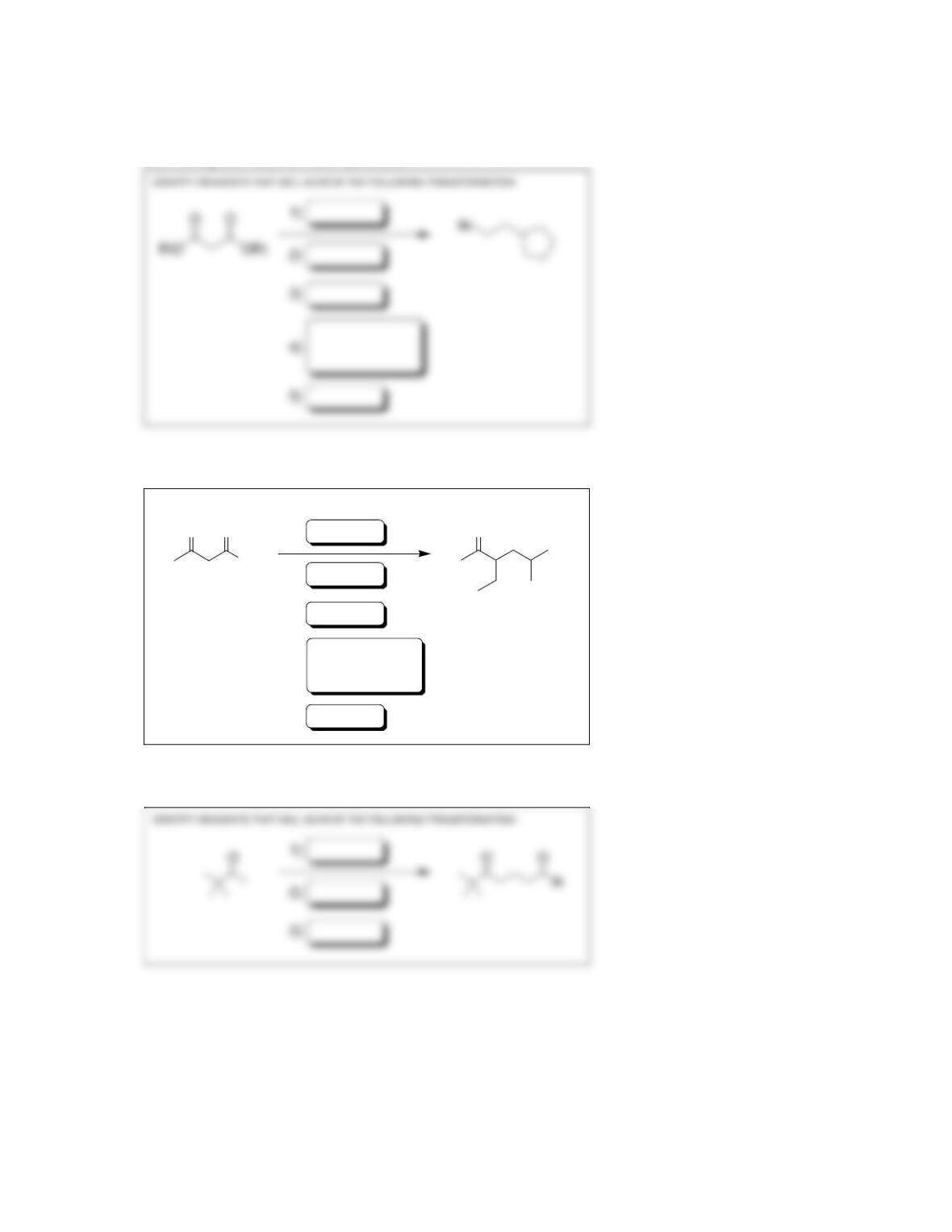
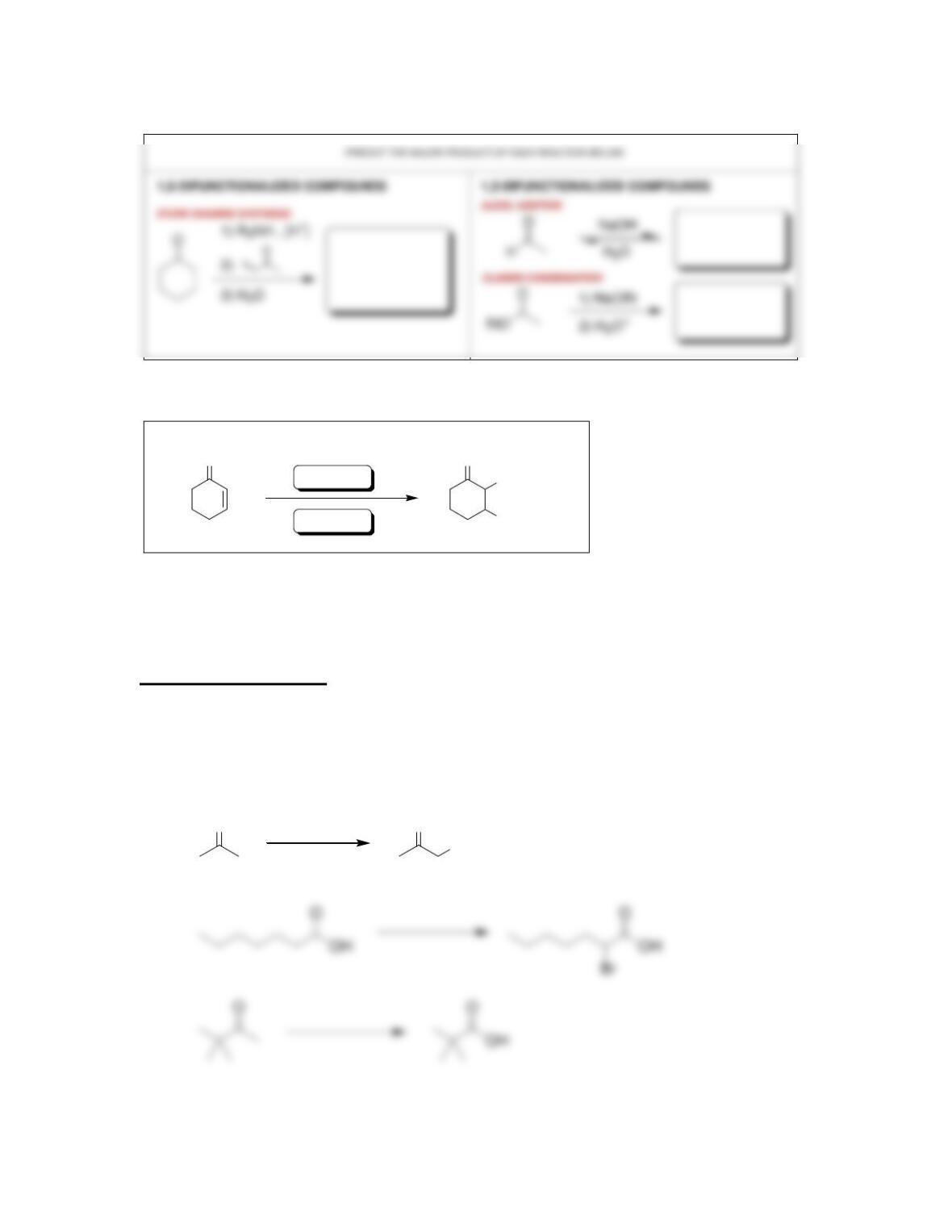
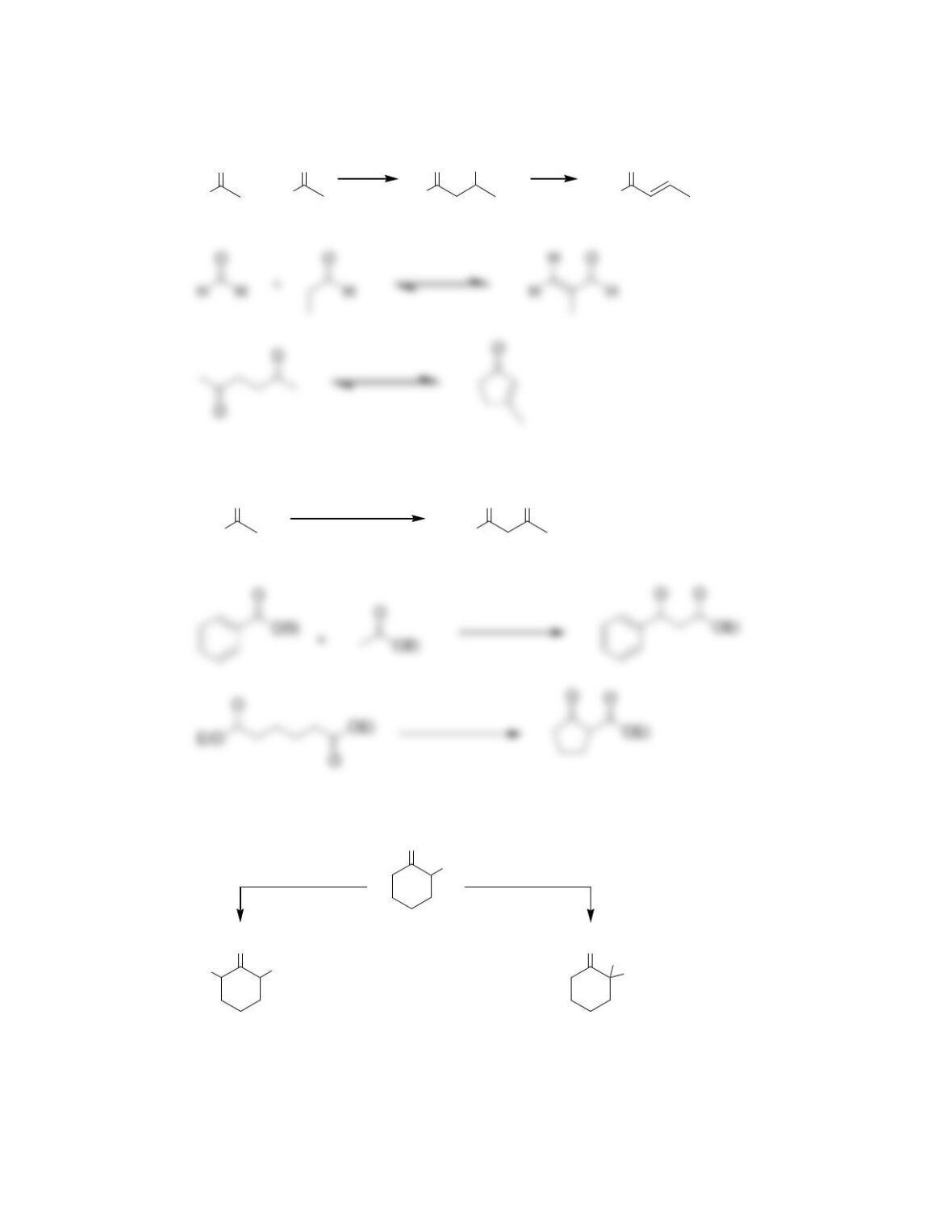
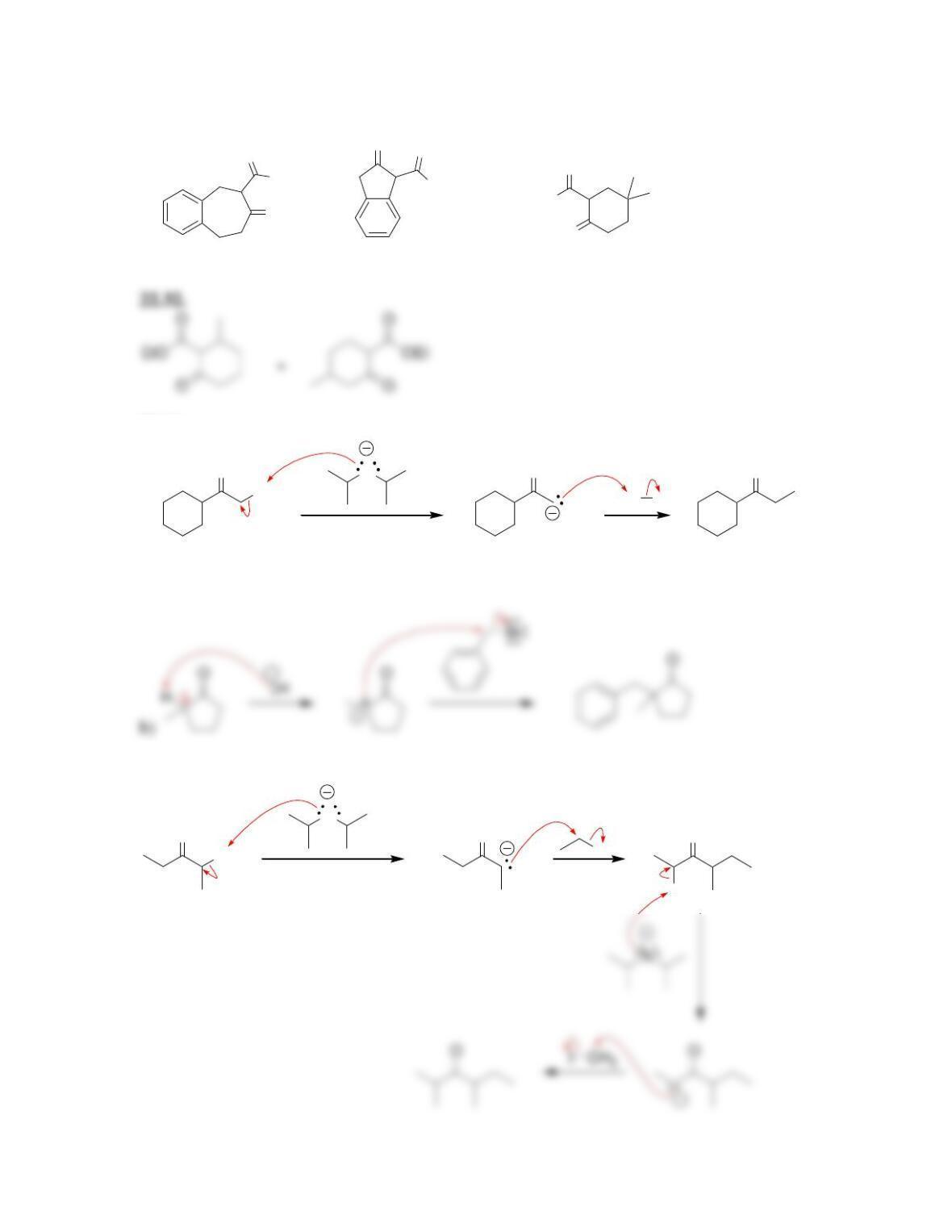
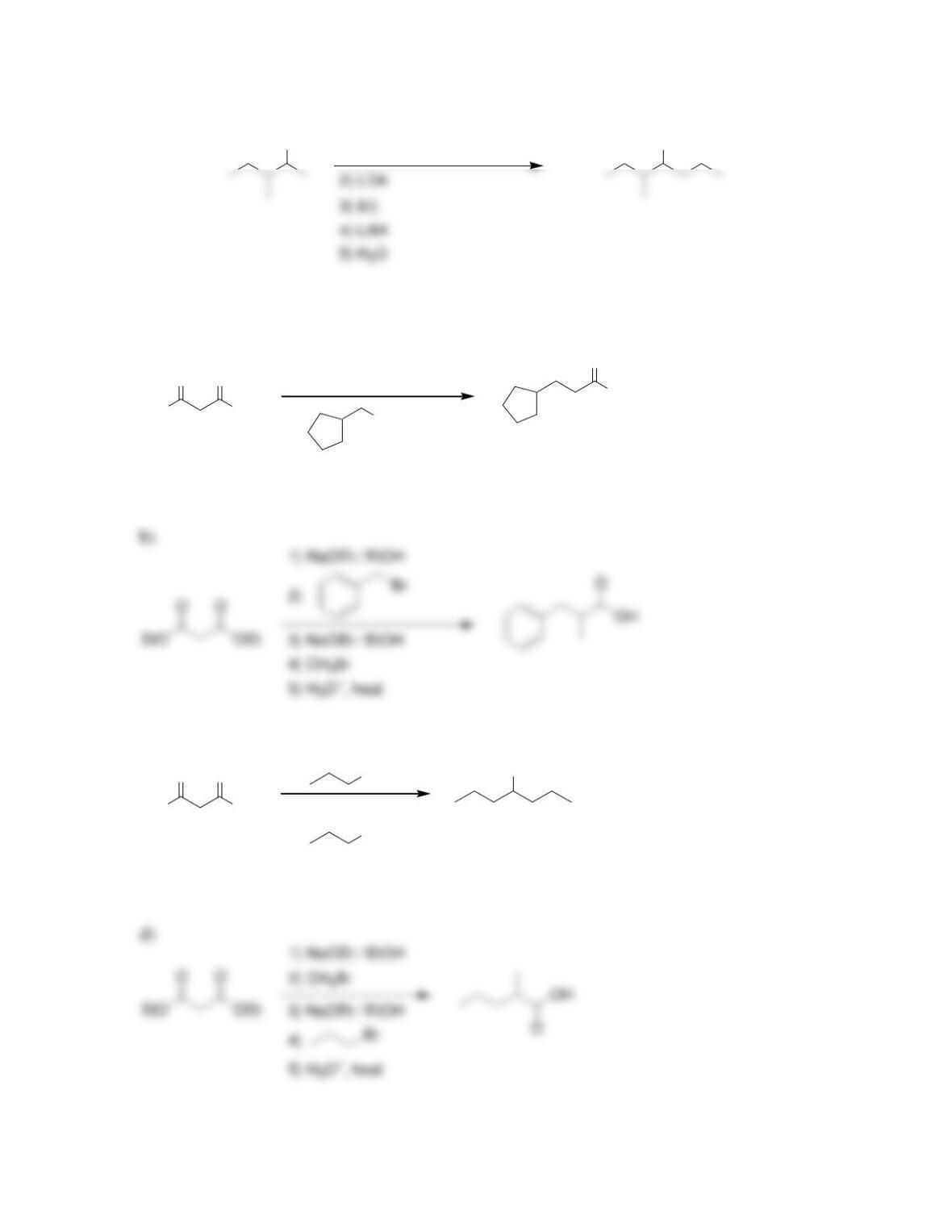
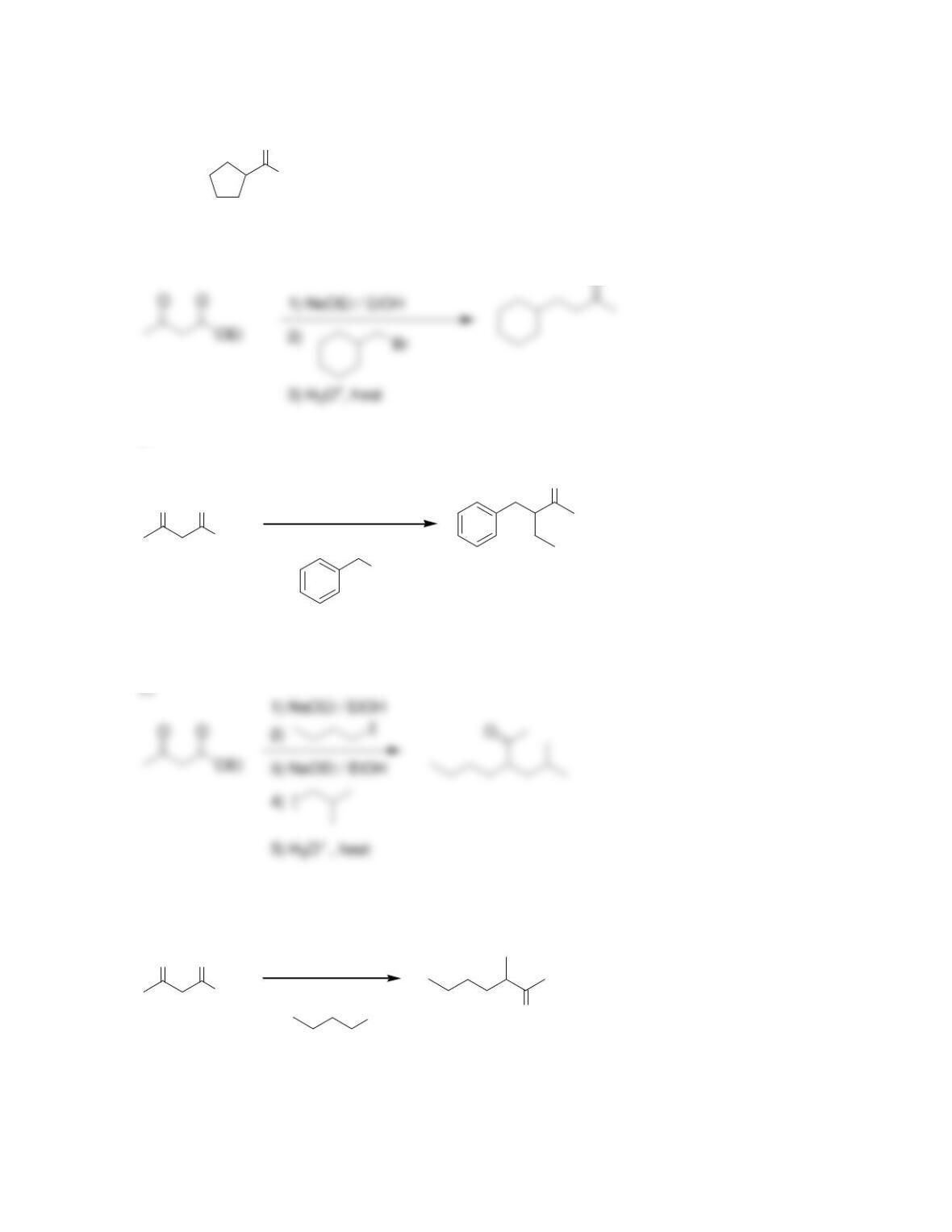
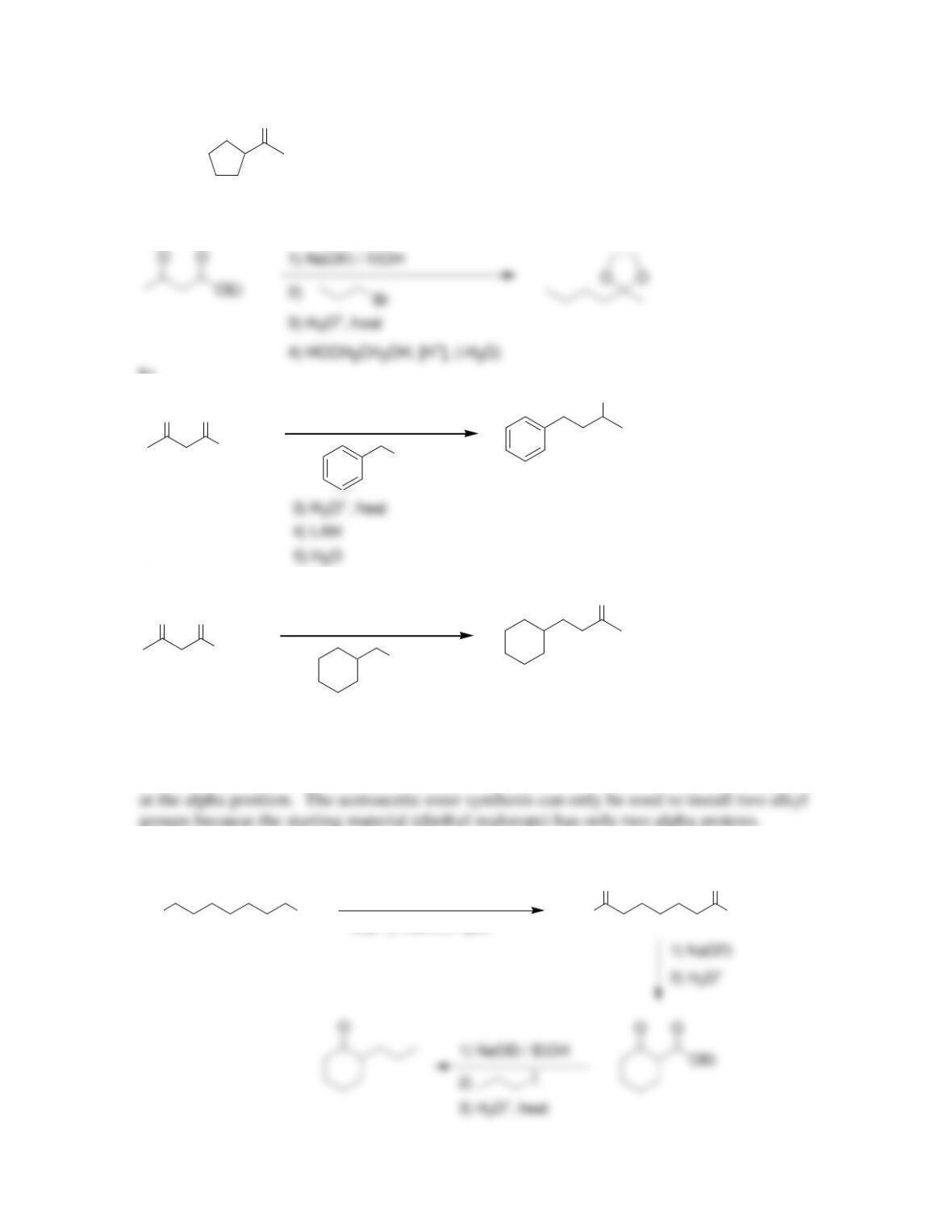
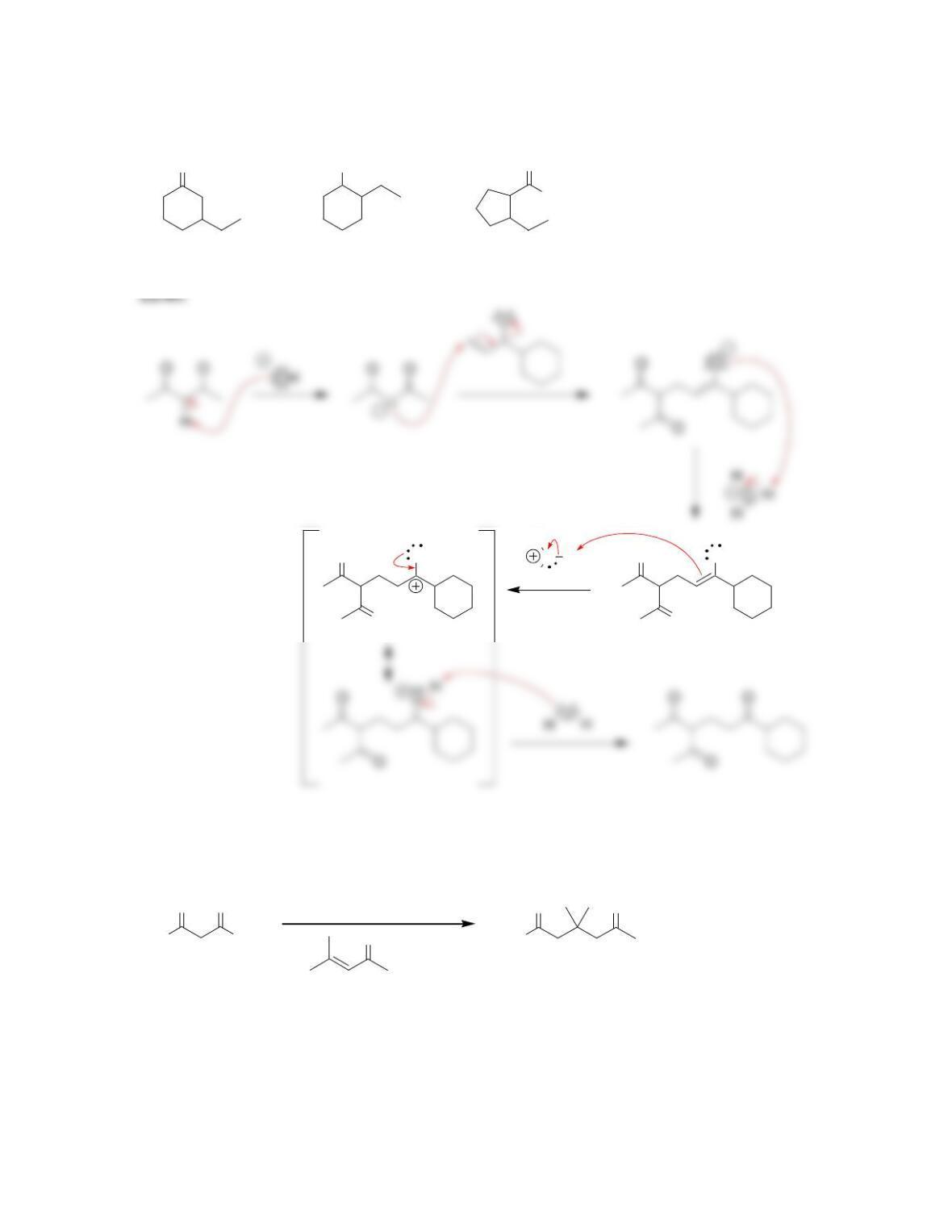
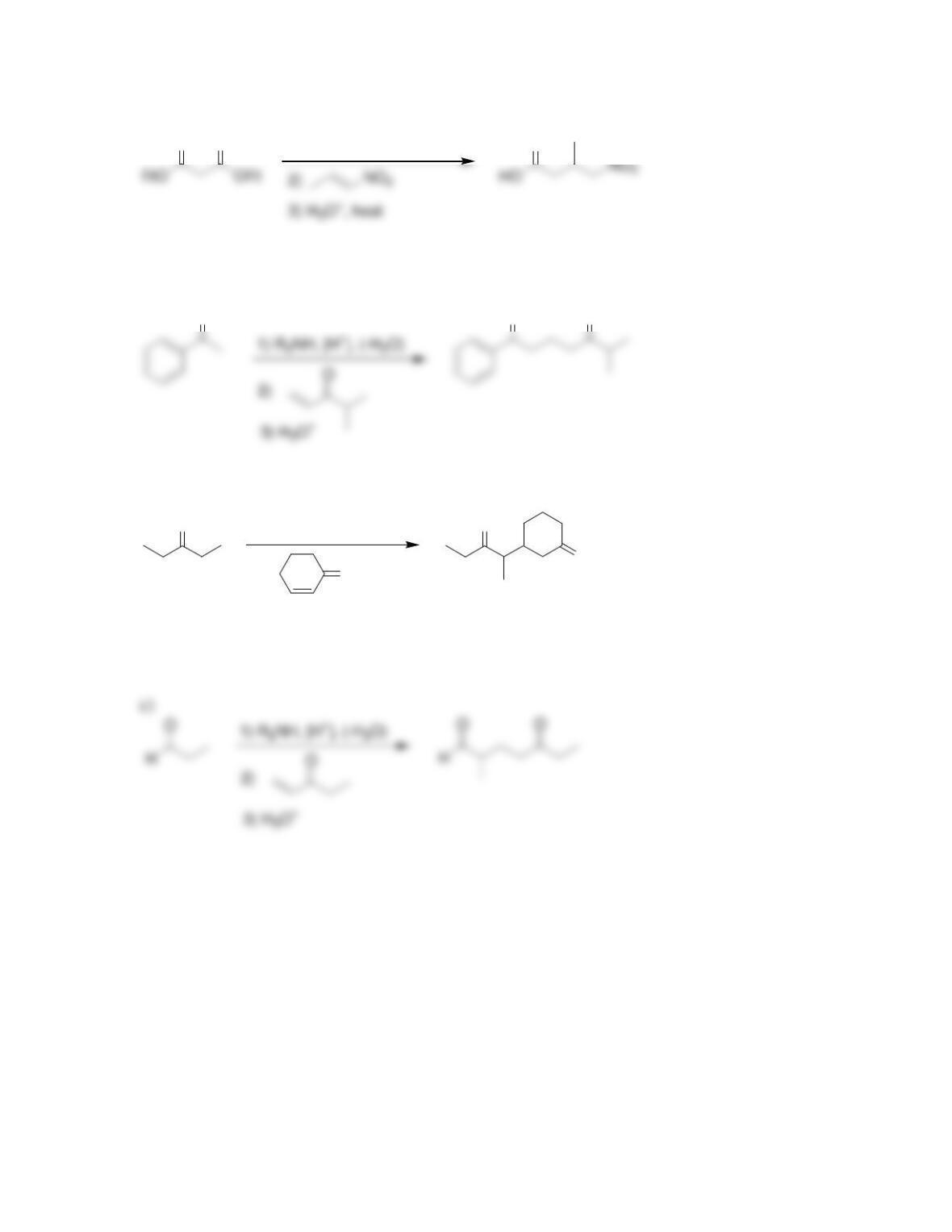
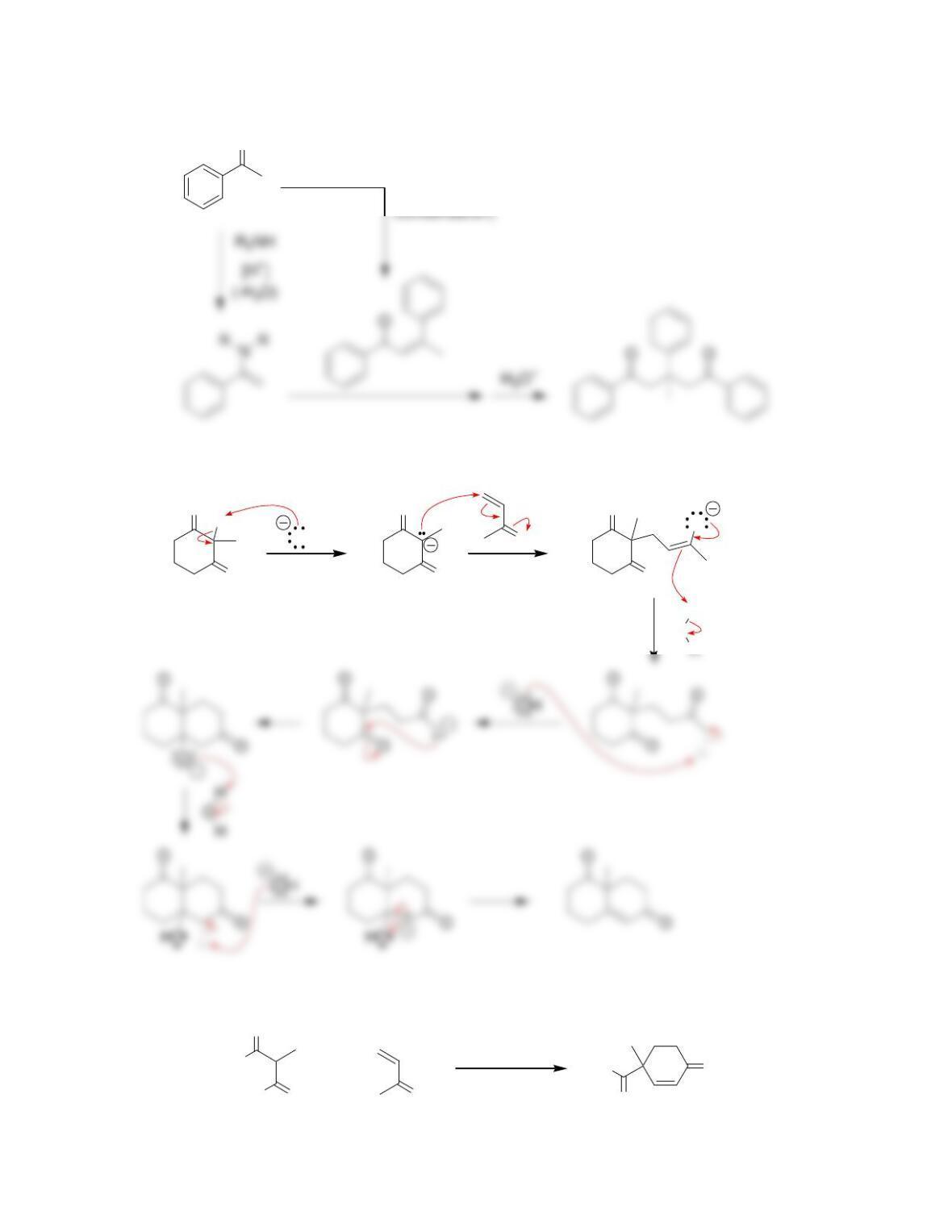
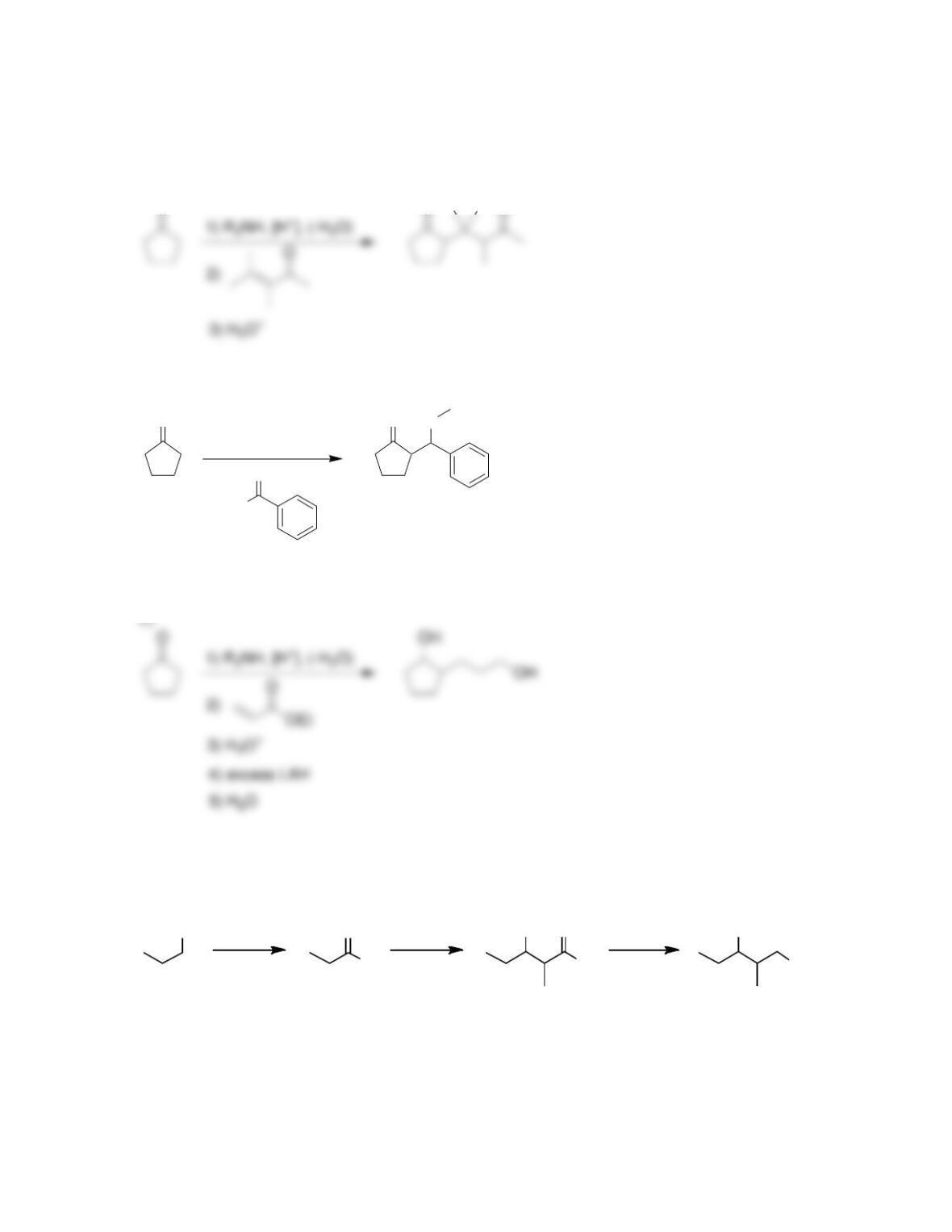
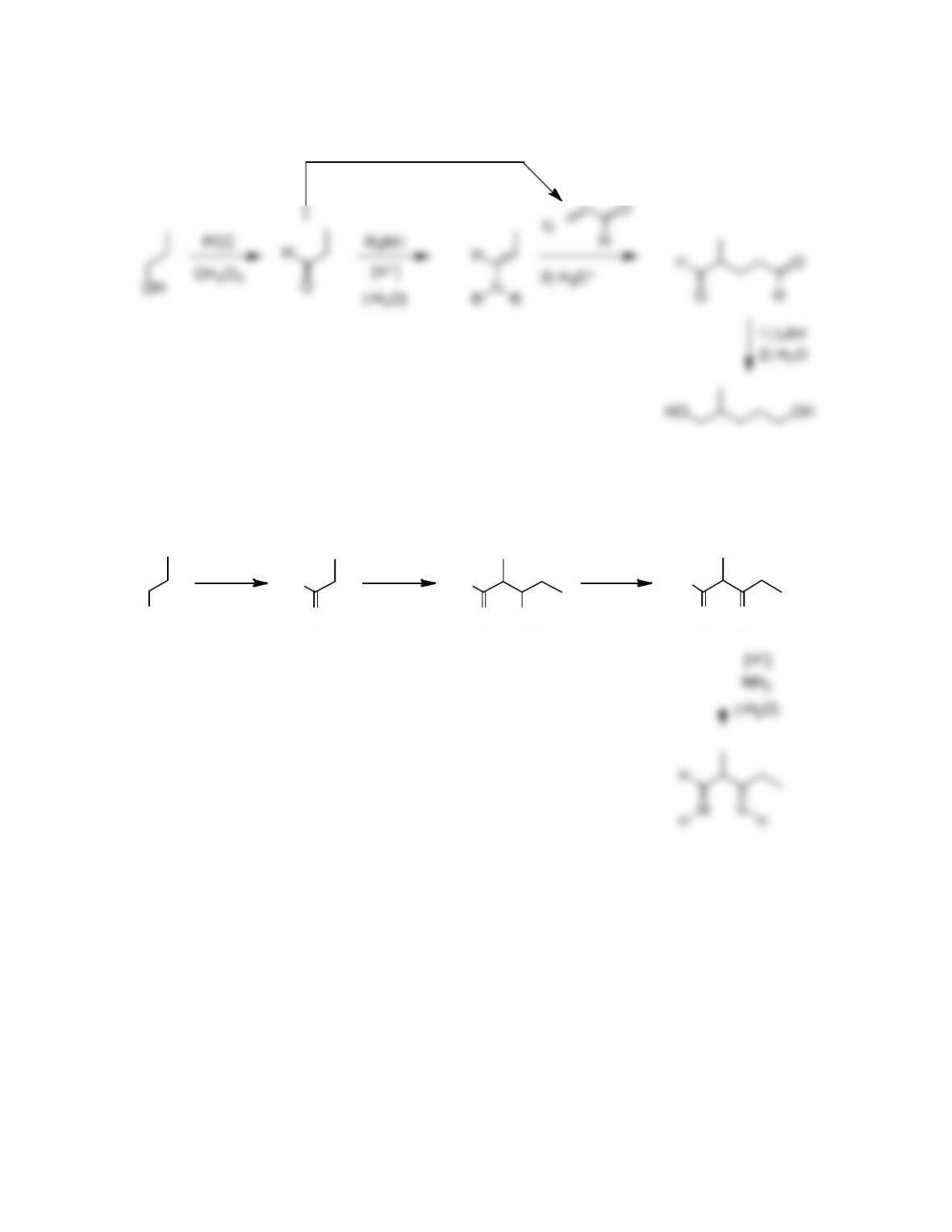
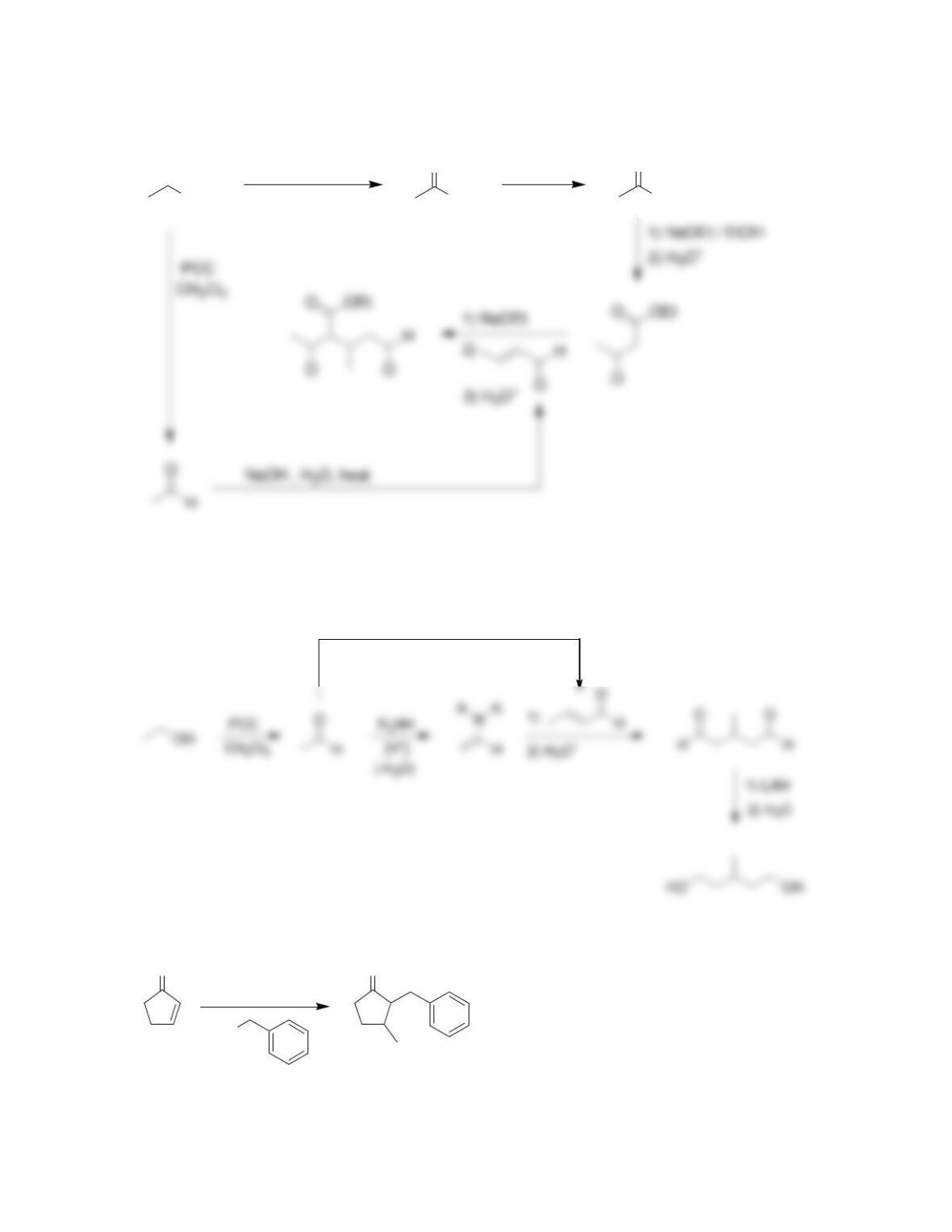
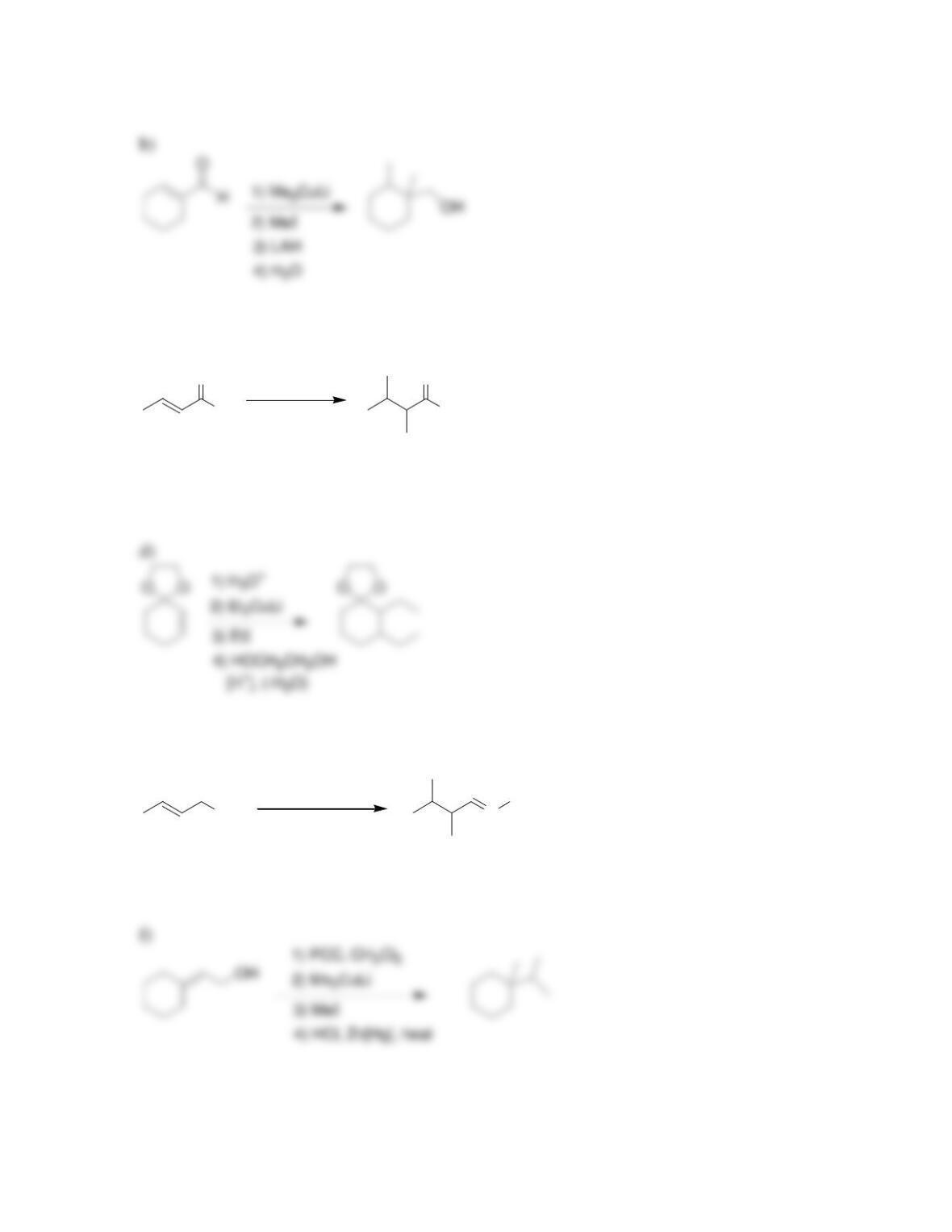
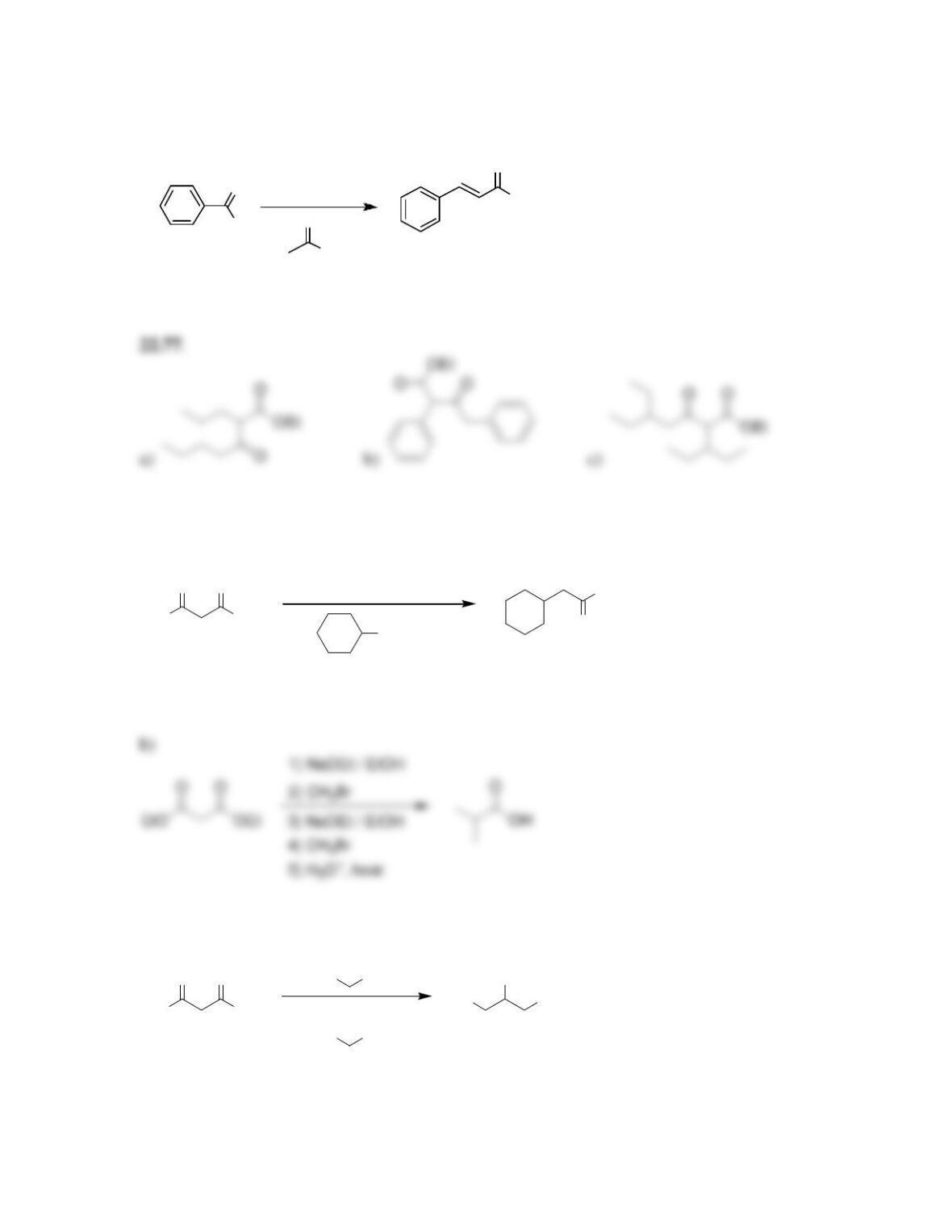
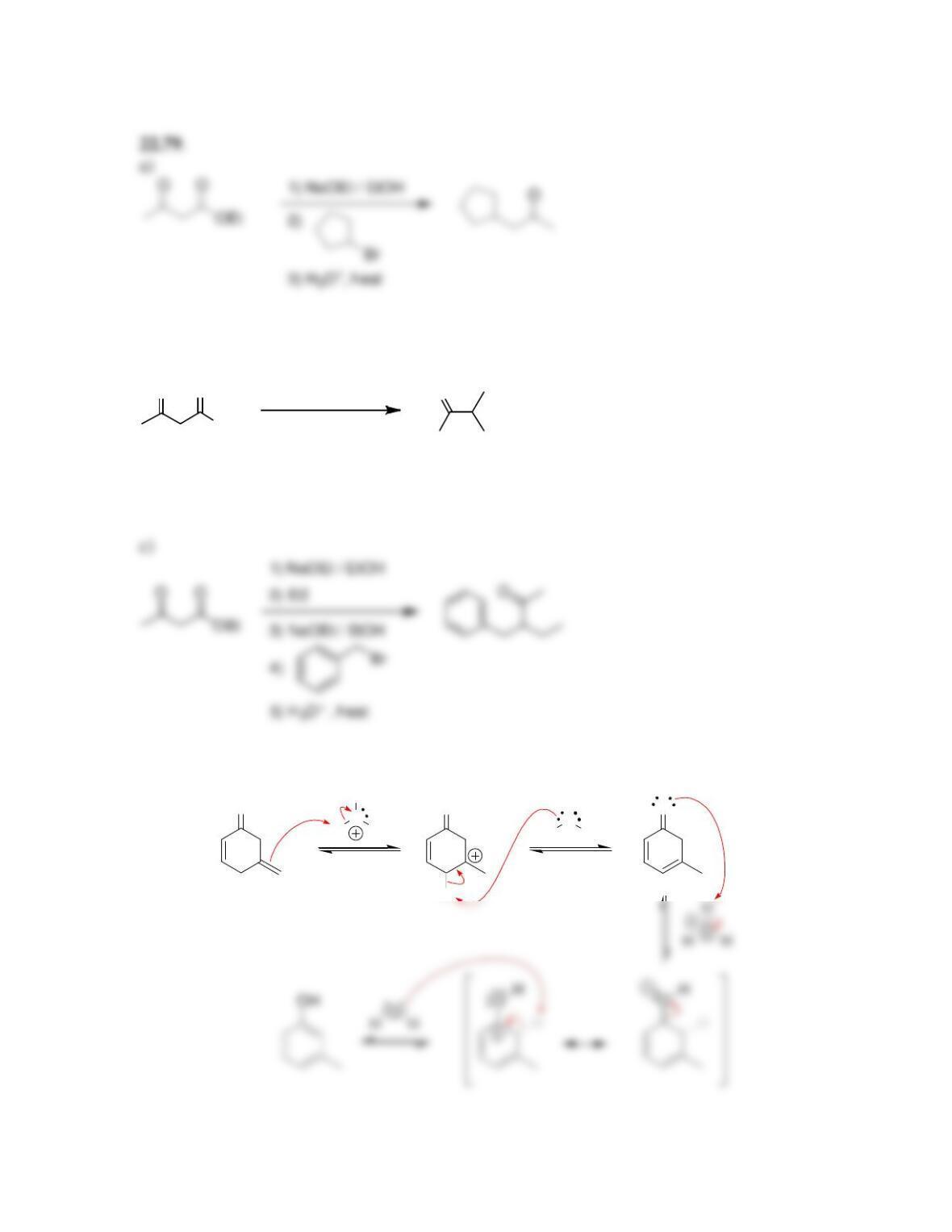
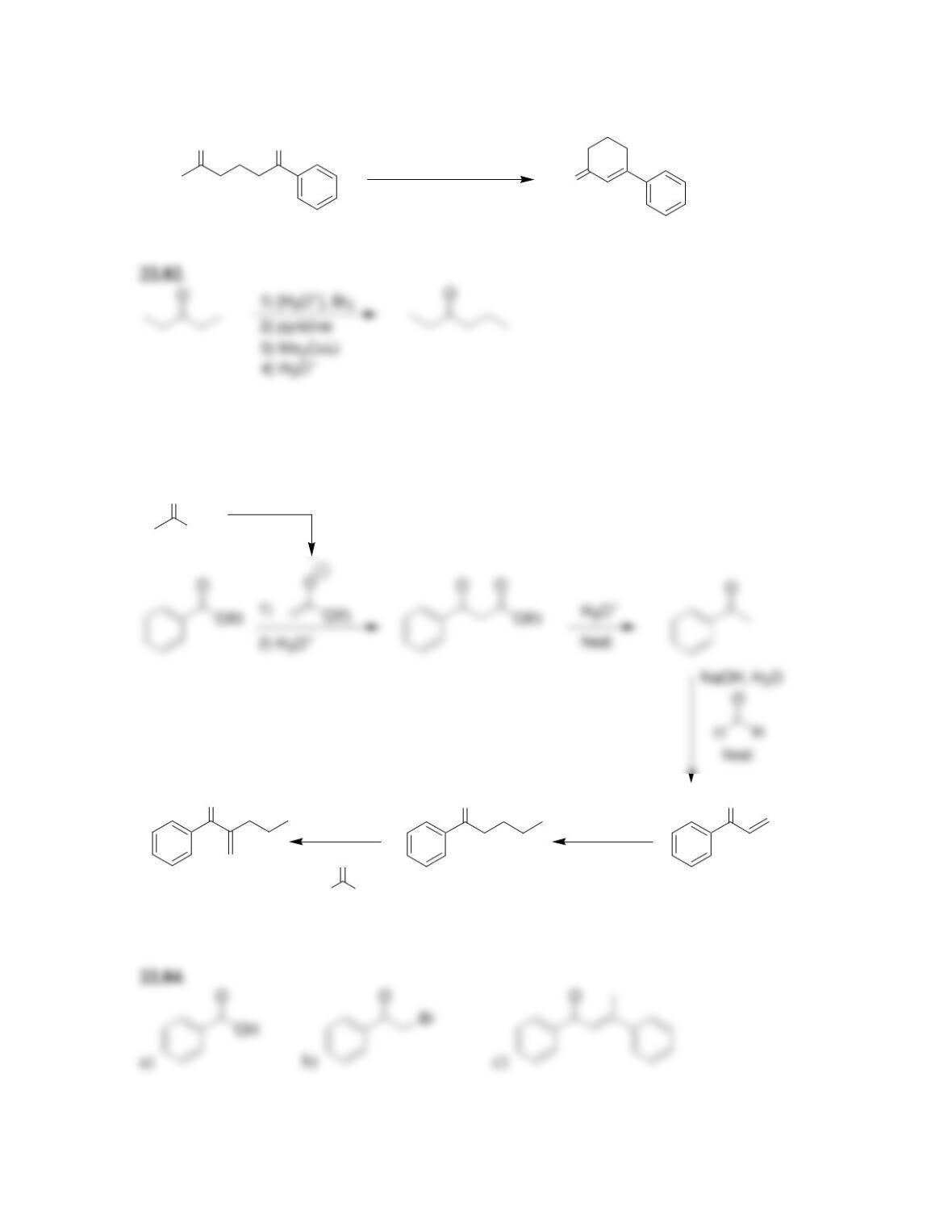
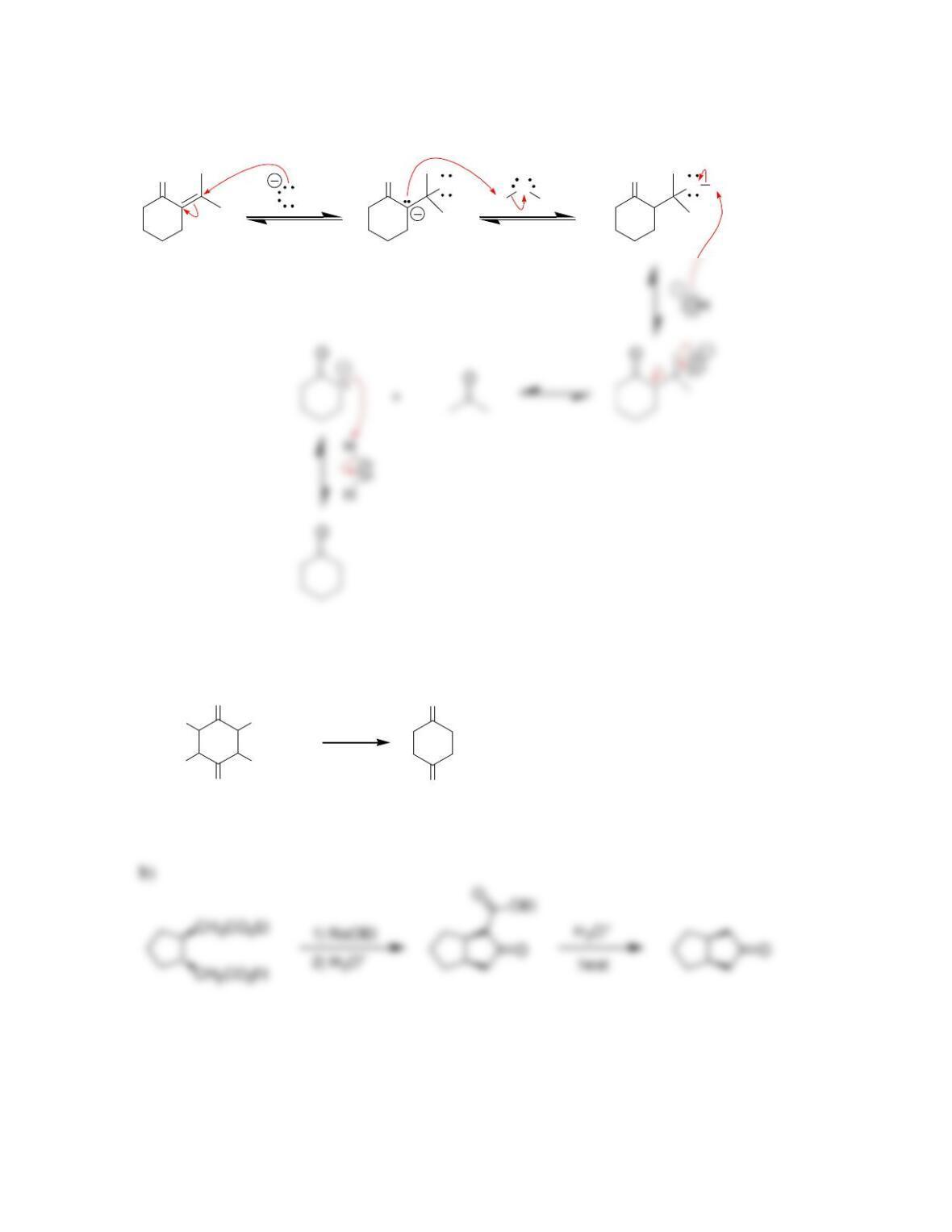
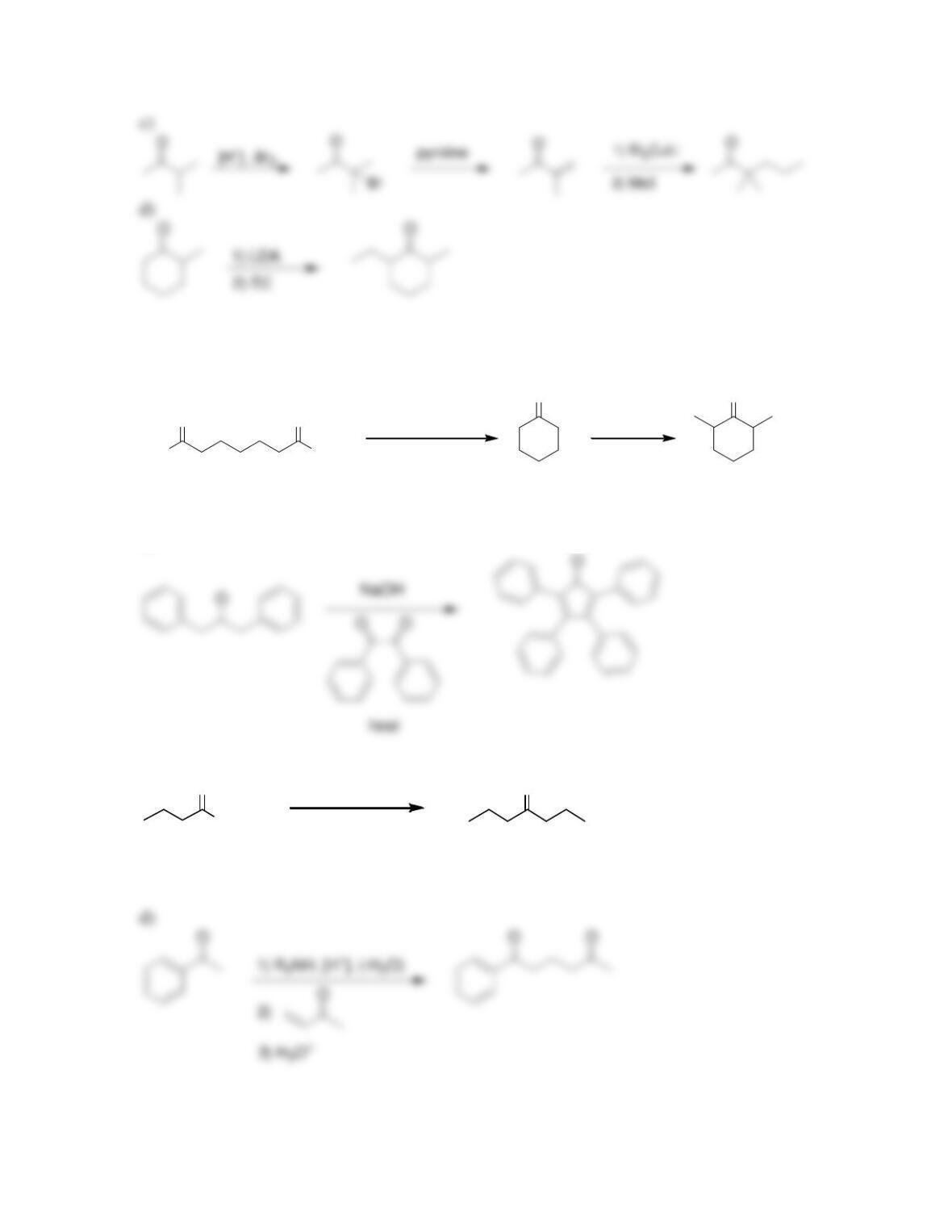
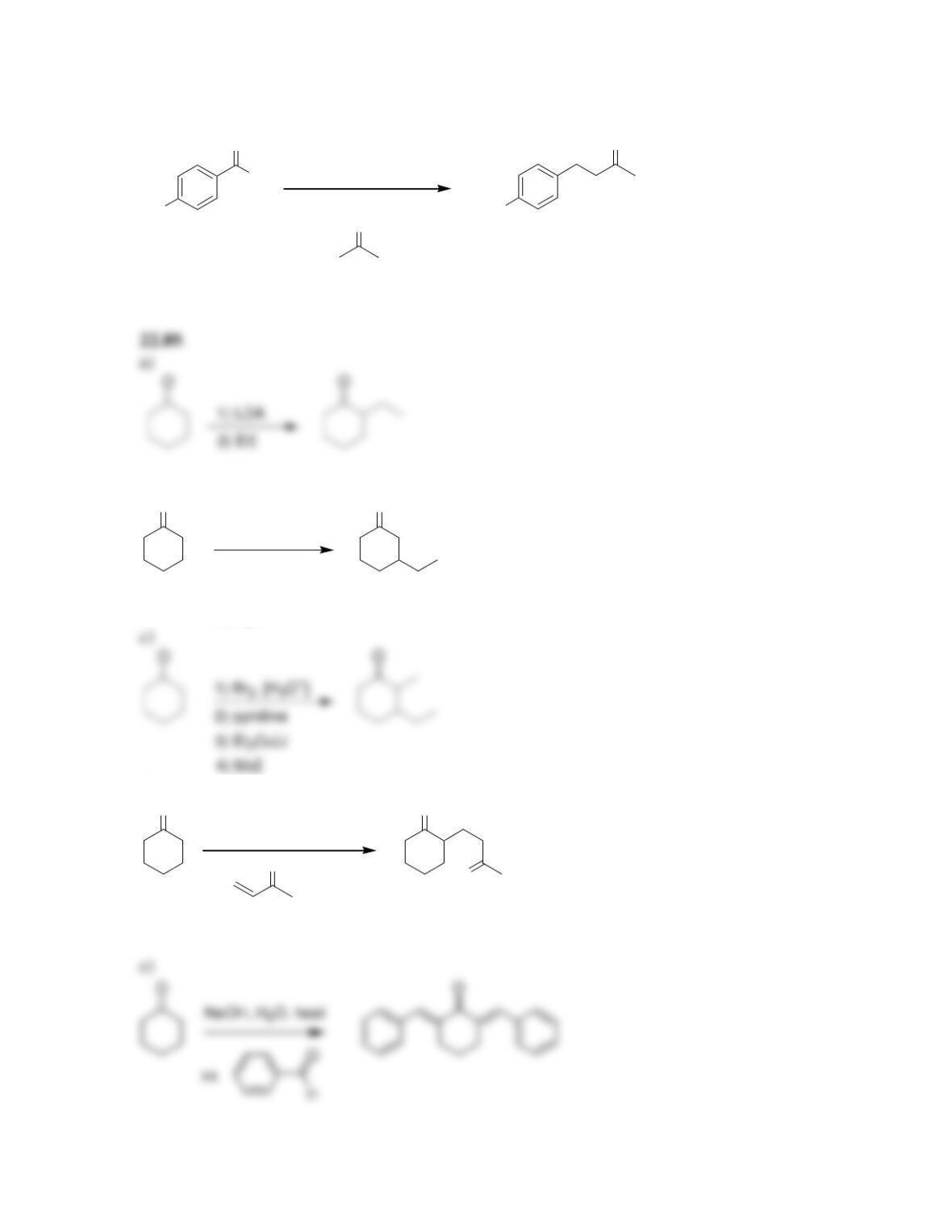
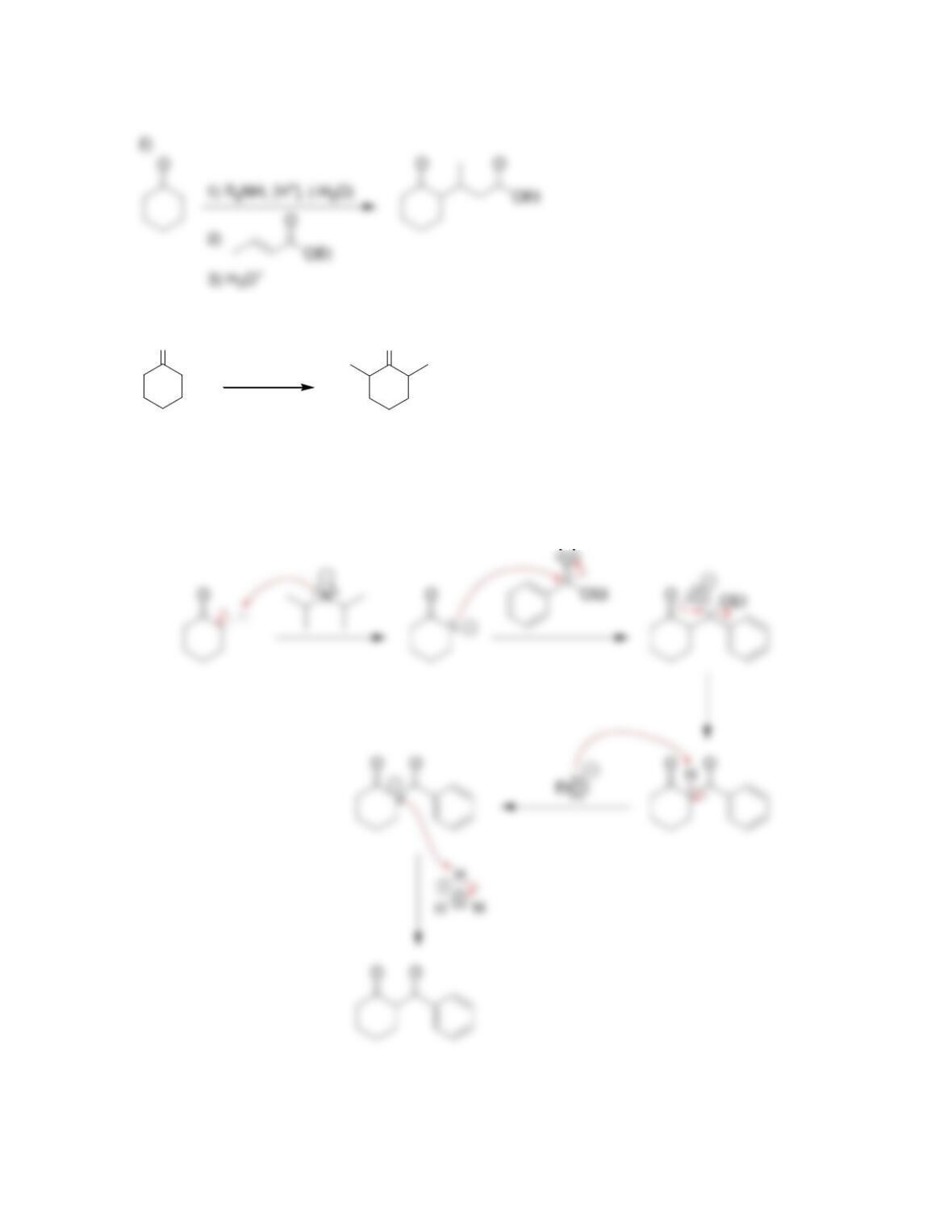
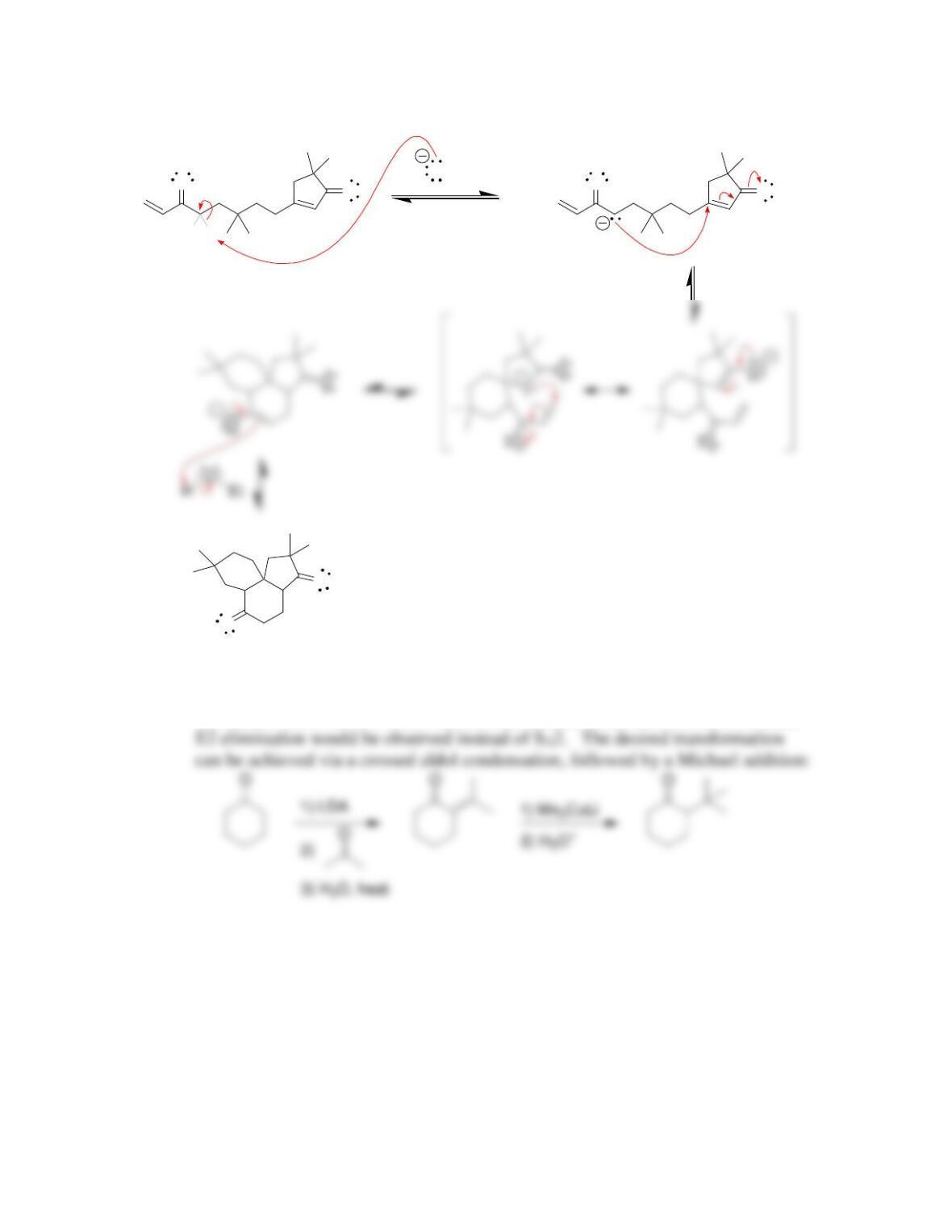
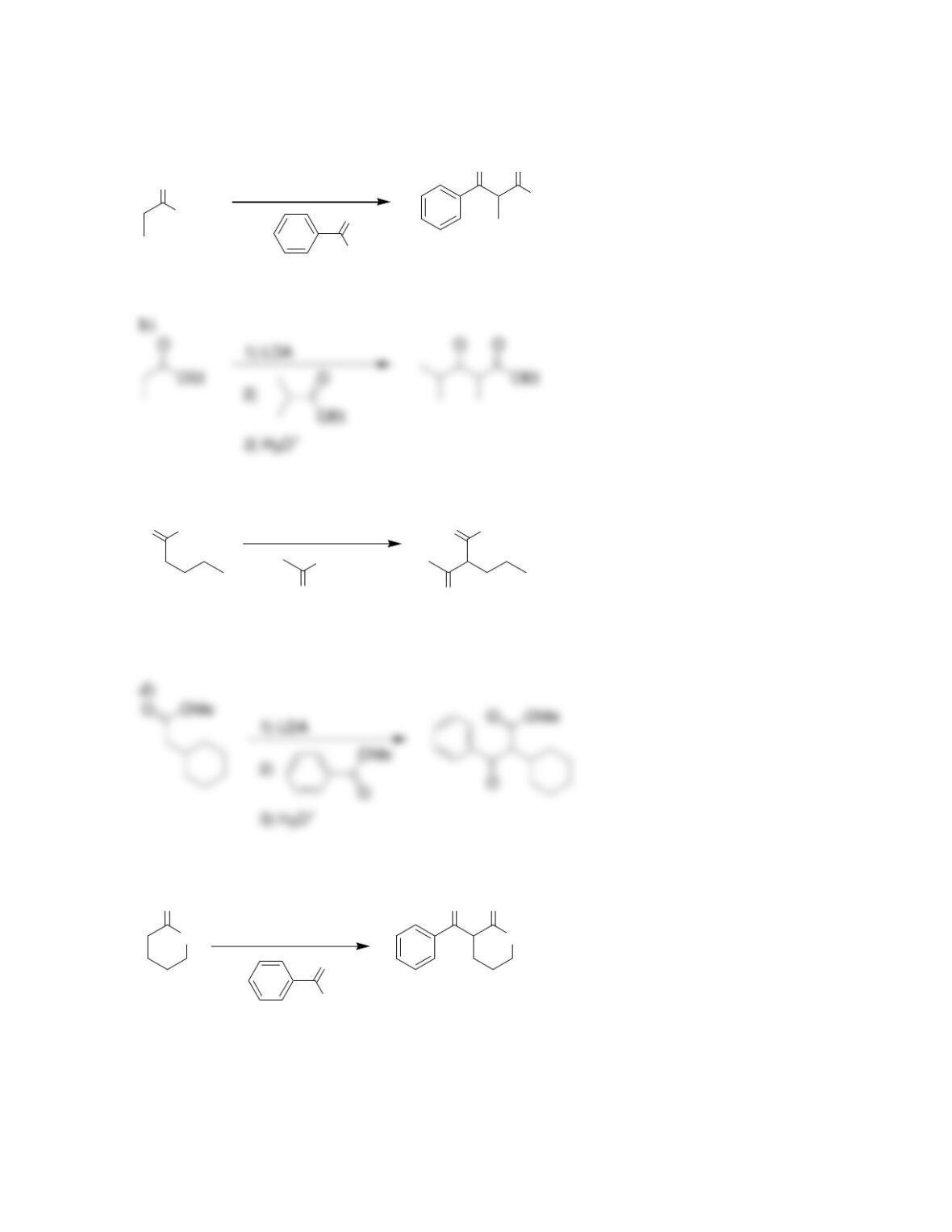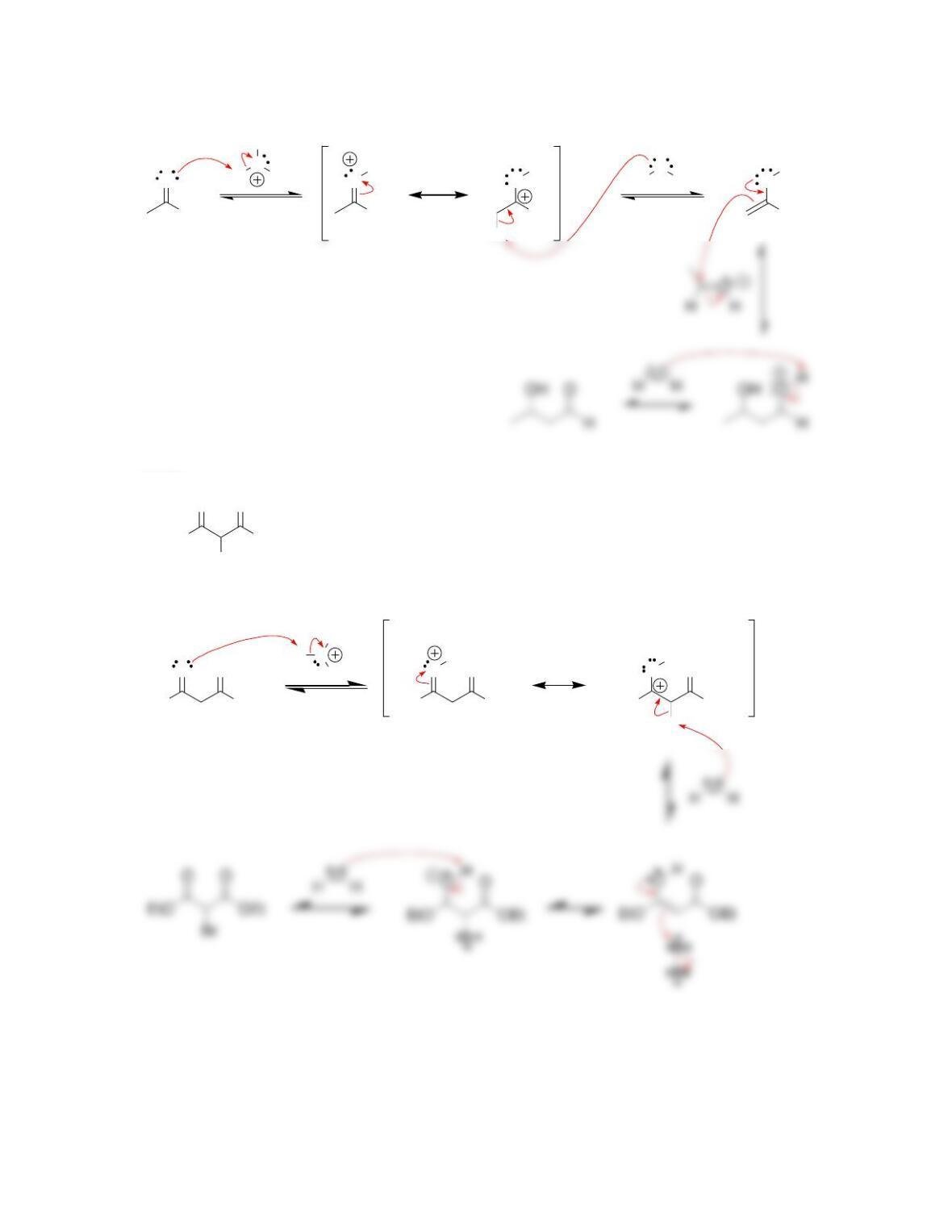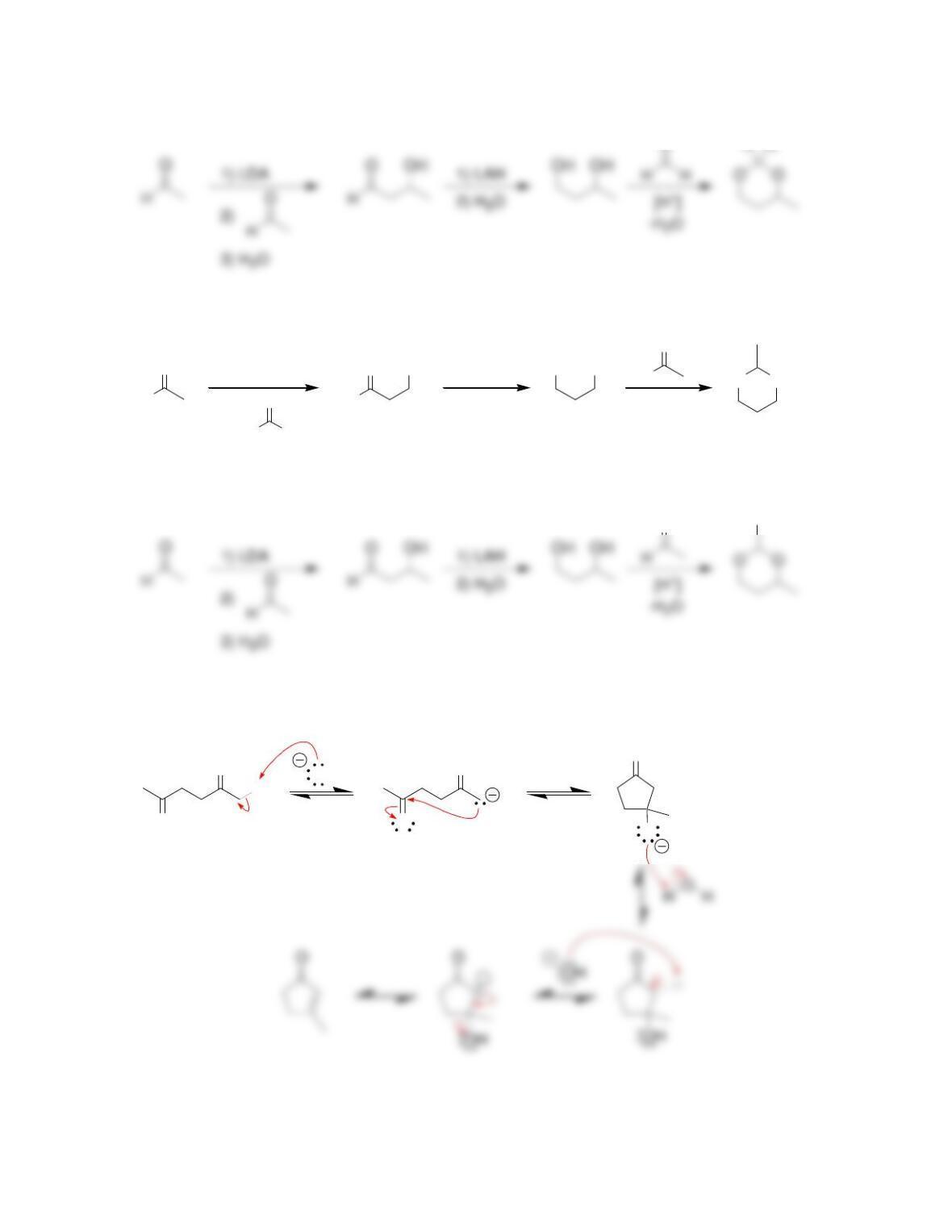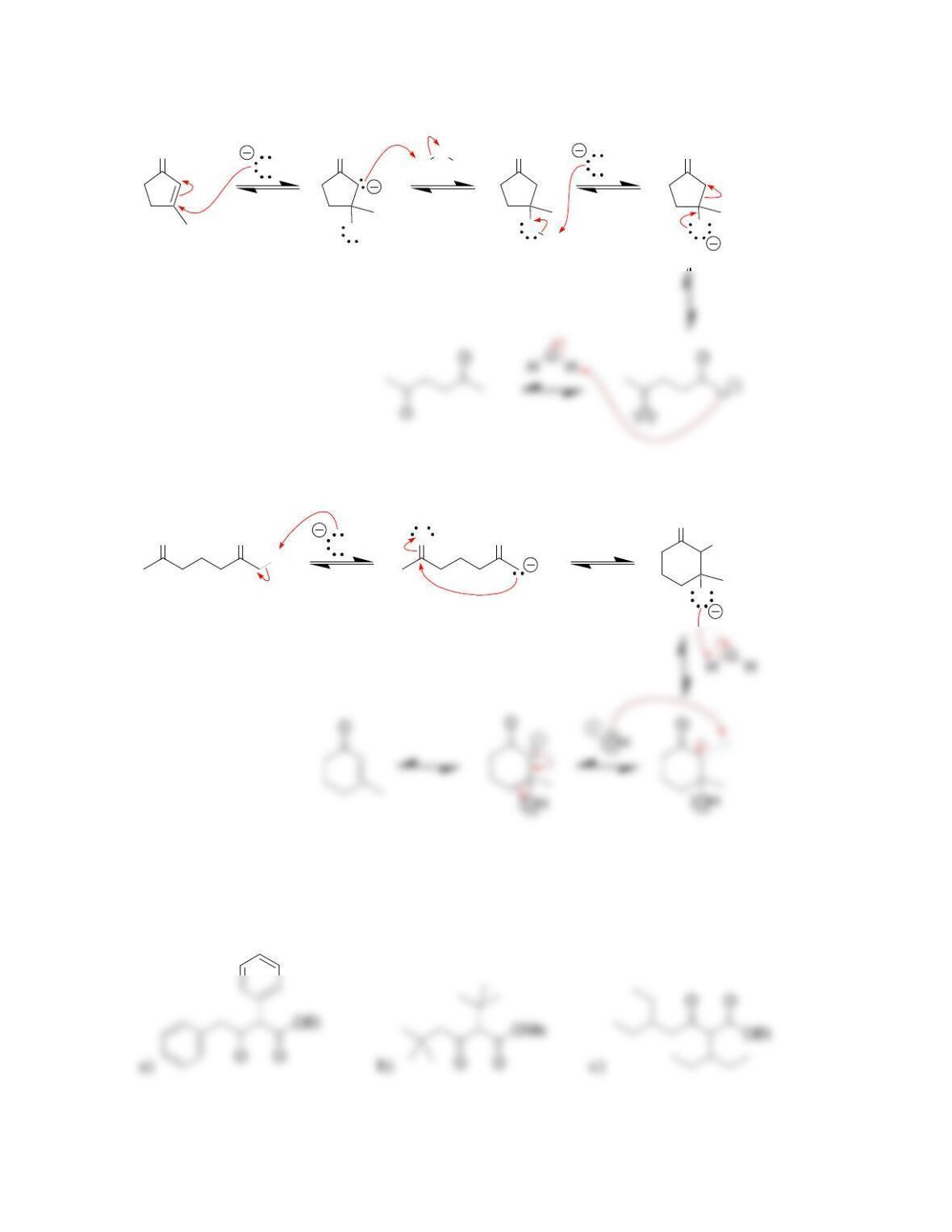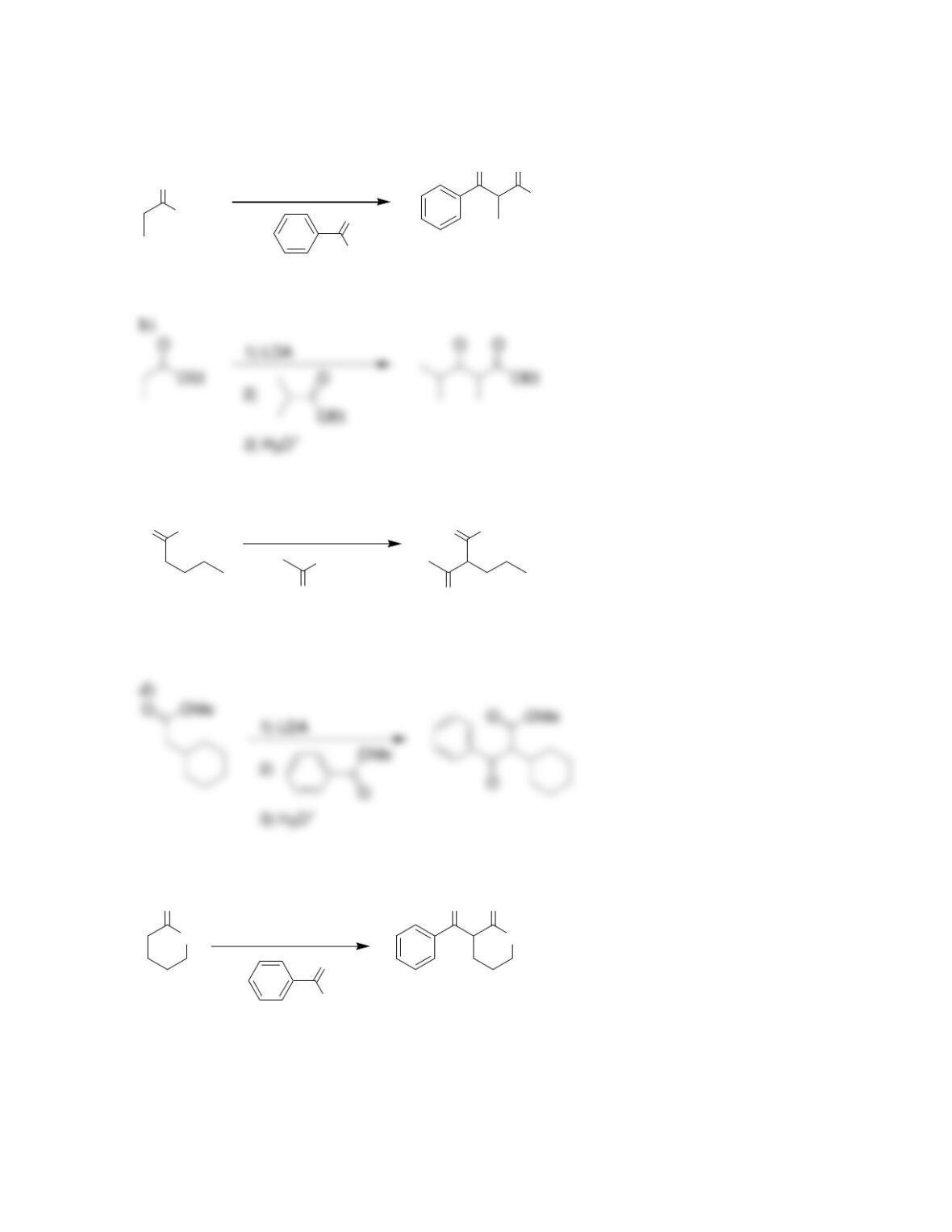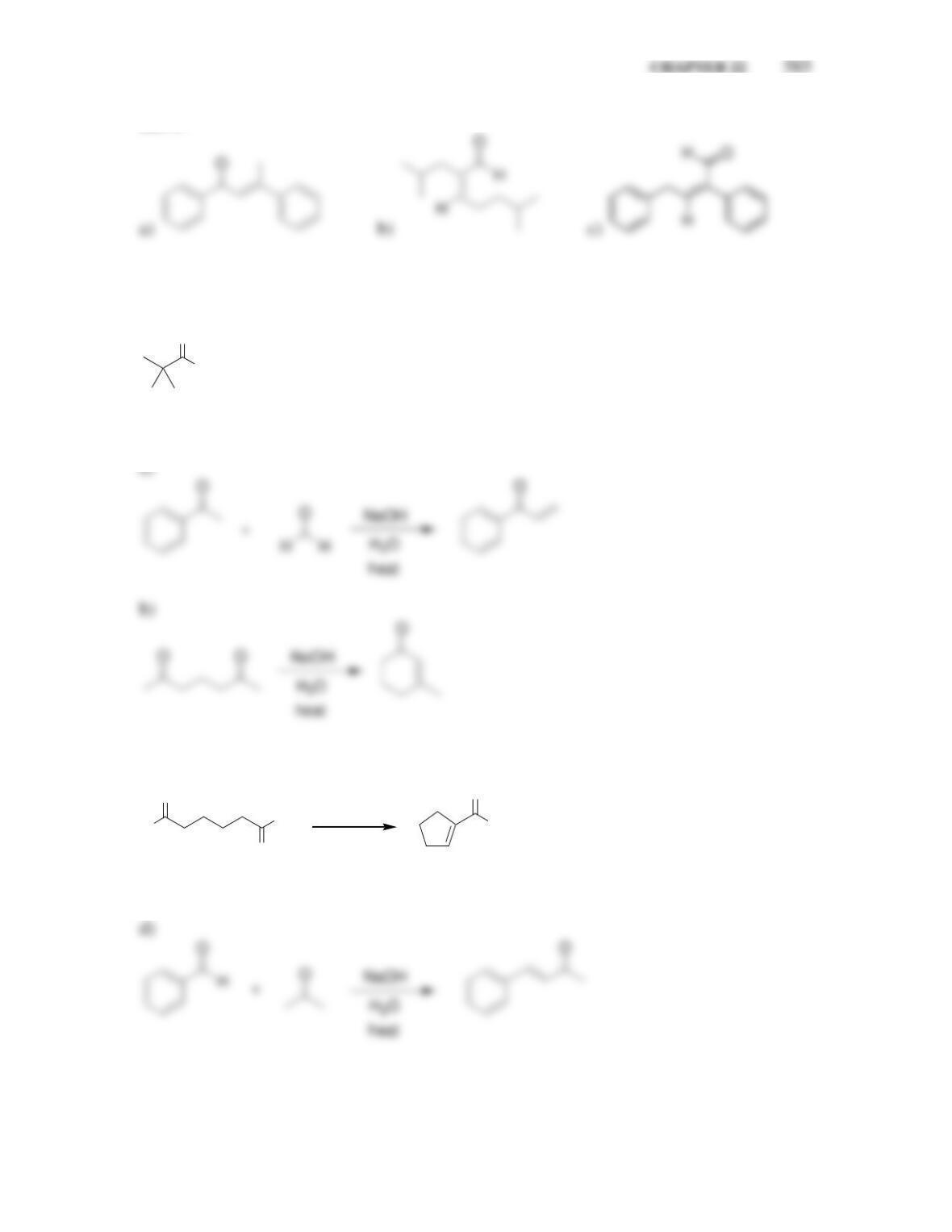Chapter 22
Alpha Carbon Chemistry: Enols and Enolates
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 22. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• When an aldehyde is treated with sodium hydroxide, an aldol addition reaction
occurs, and the product is a ________________________________.
• For most simple aldehydes, the position of equilibrium favors the aldol product.
For most ketones, the reverse process, called a ______-aldol reaction is favored.
• When an aldehyde is heated in aqueous sodium hydroxide, an aldol ___________
reaction occurs, and the product is an ___________________________.
• For unsymmetrical ketones, reactions with _____ at low temperature favor
formation of the kinetic enolate, while reactions with ______ at room temperature
favor the thermodynamic enolate.
• When LDA is used with an unsymmetrical ketone, alkylation occurs at the
__________________ position.