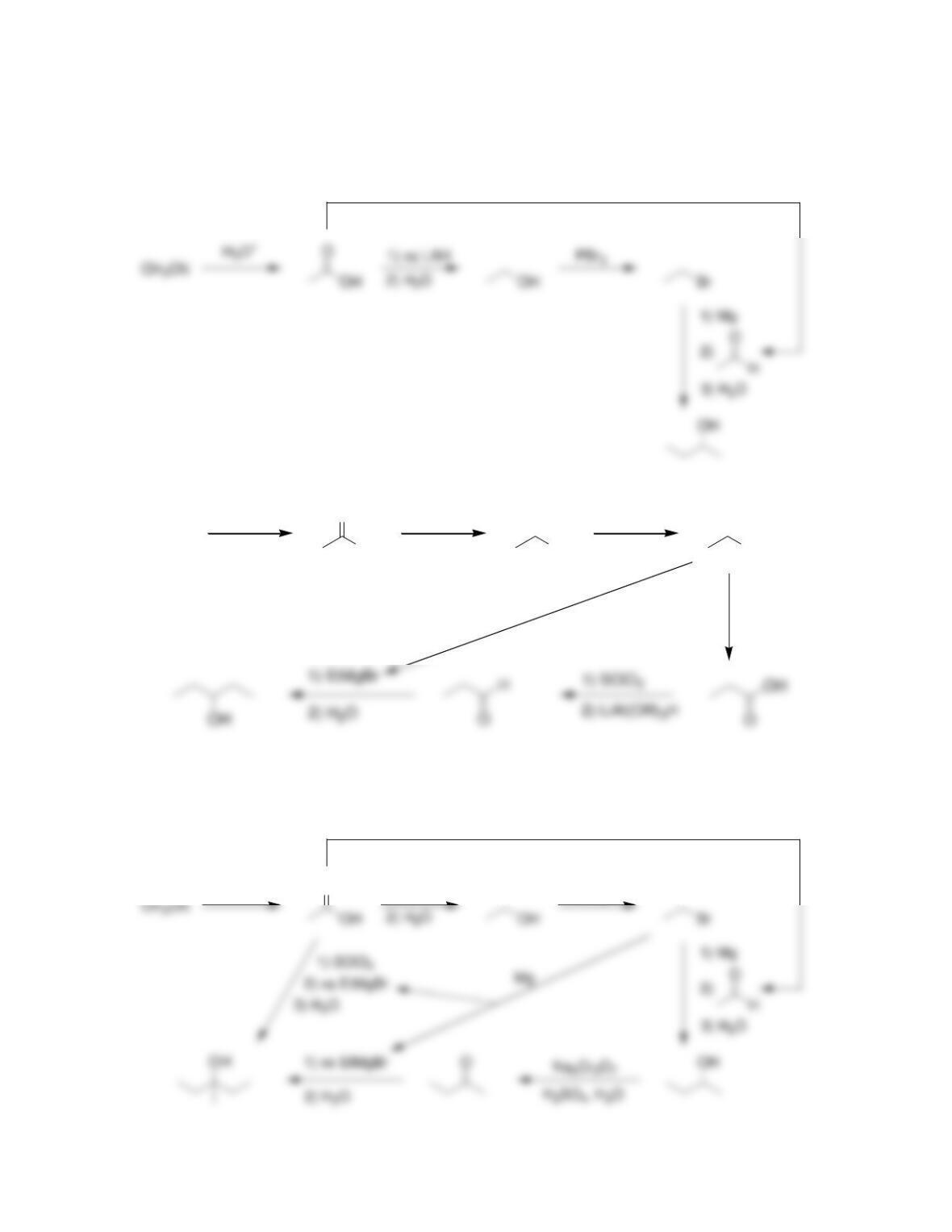
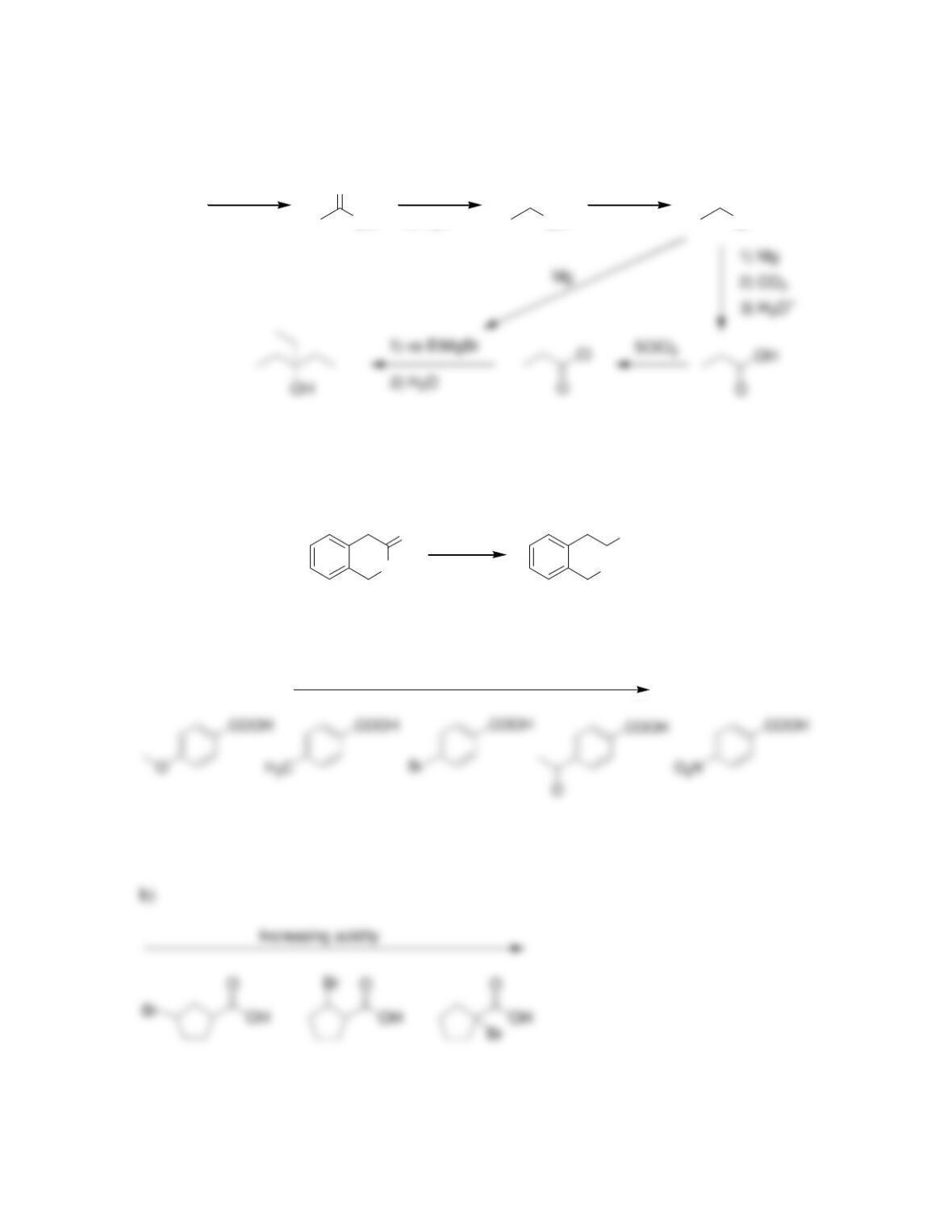
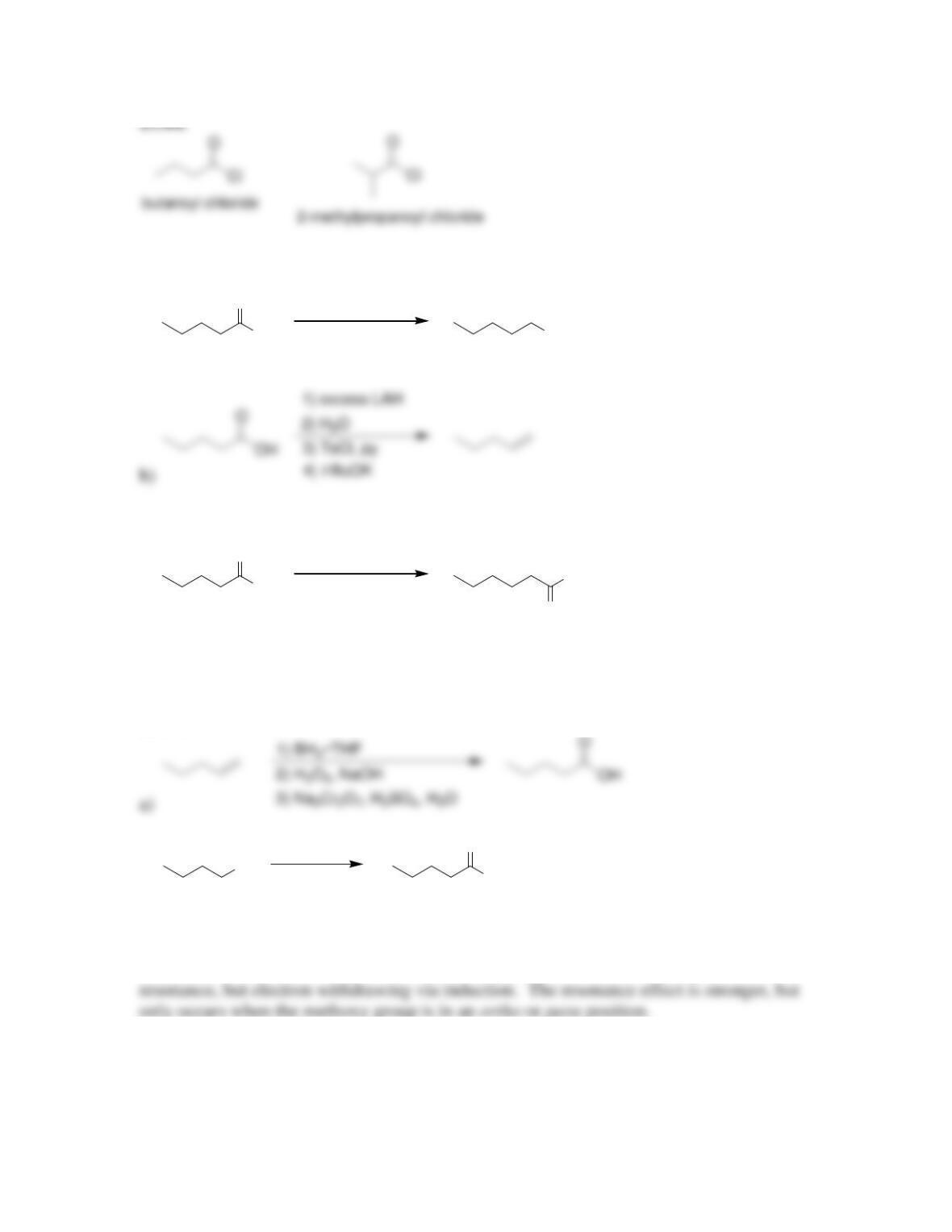
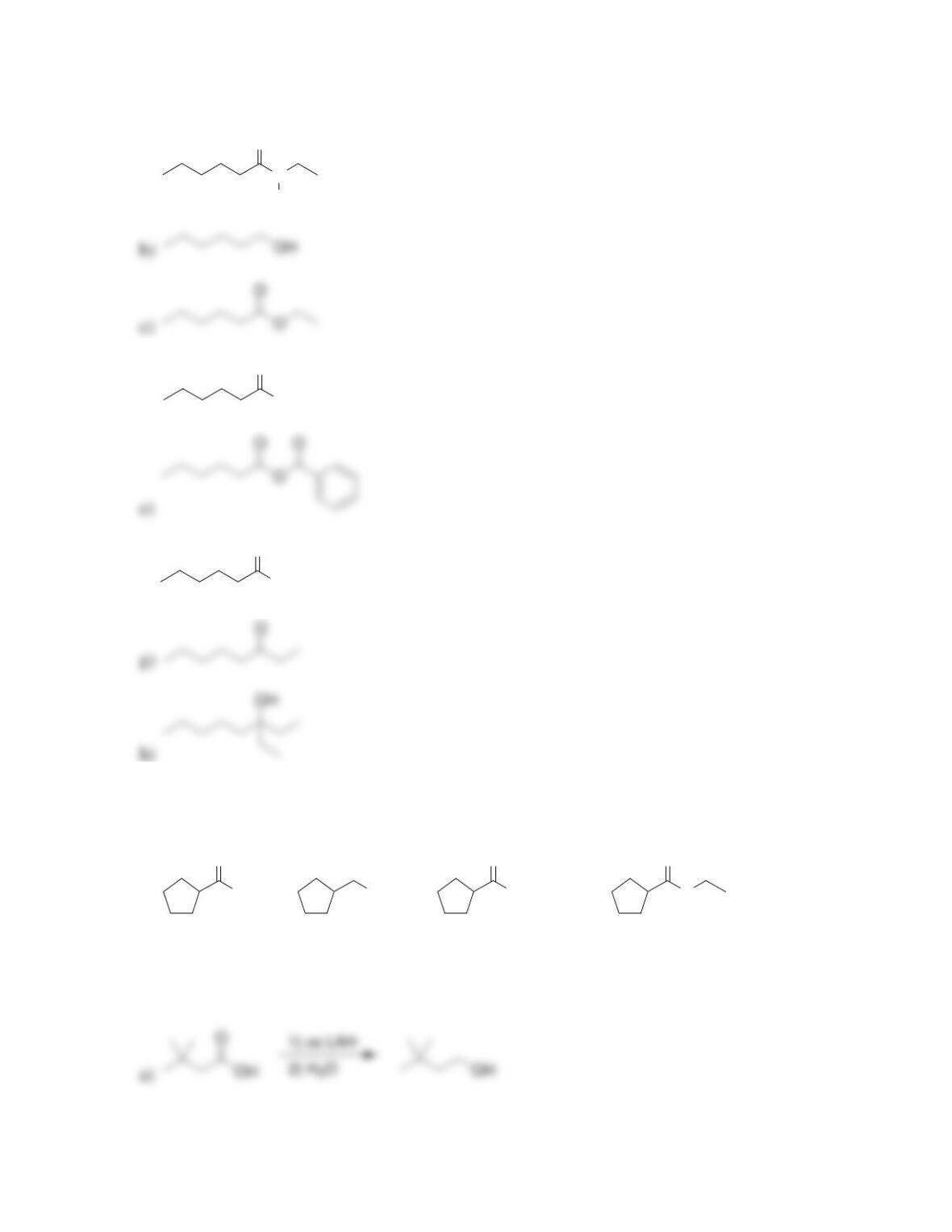
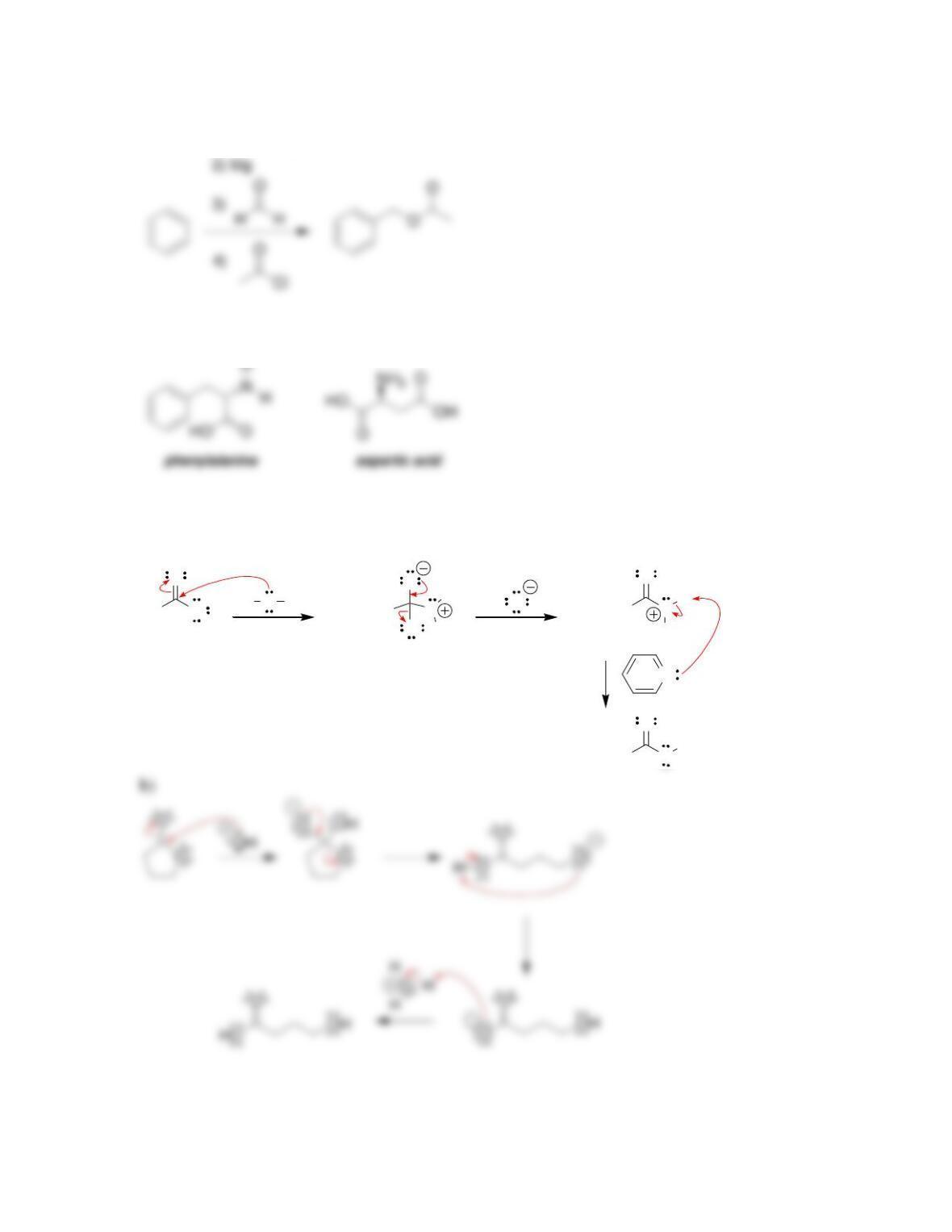Chapter 21
Carboxylic Acids and Their Derivatives
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 21. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• Carboxylic acid derivatives exhibit the same ________ state as carboxylic acids.
• Carboxylic acid derivatives differ in reactivity, with _____________ being the
most reactive and __________ the least reactive.
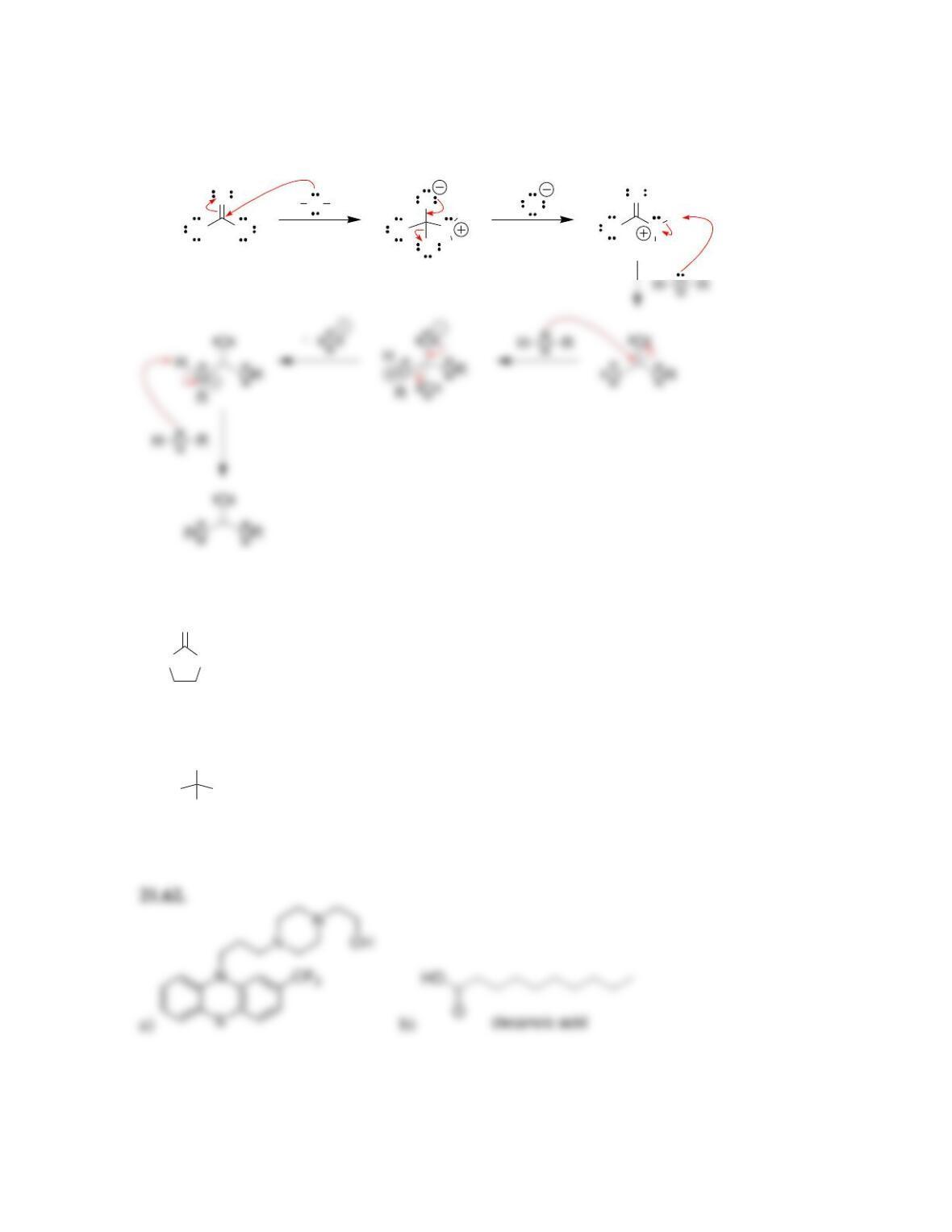
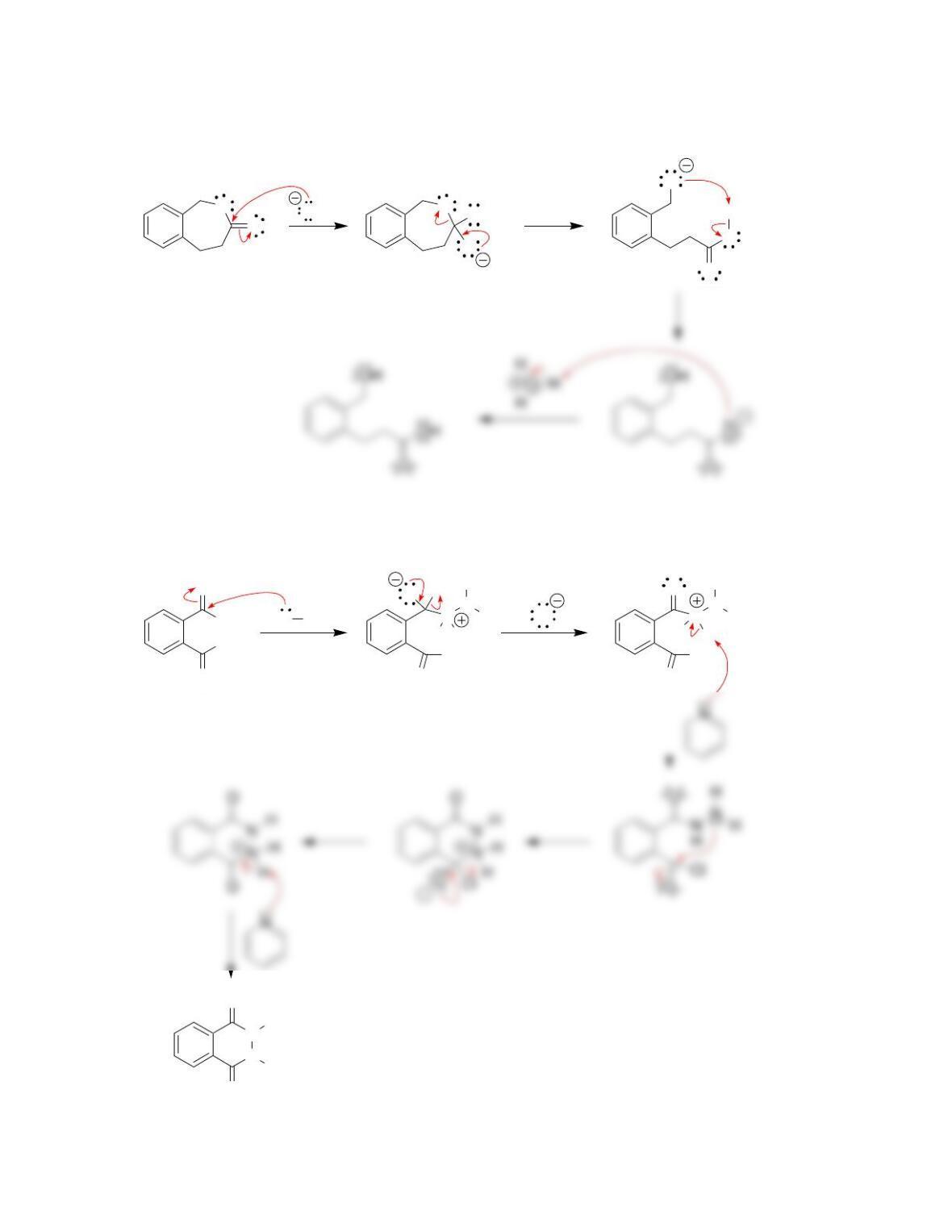
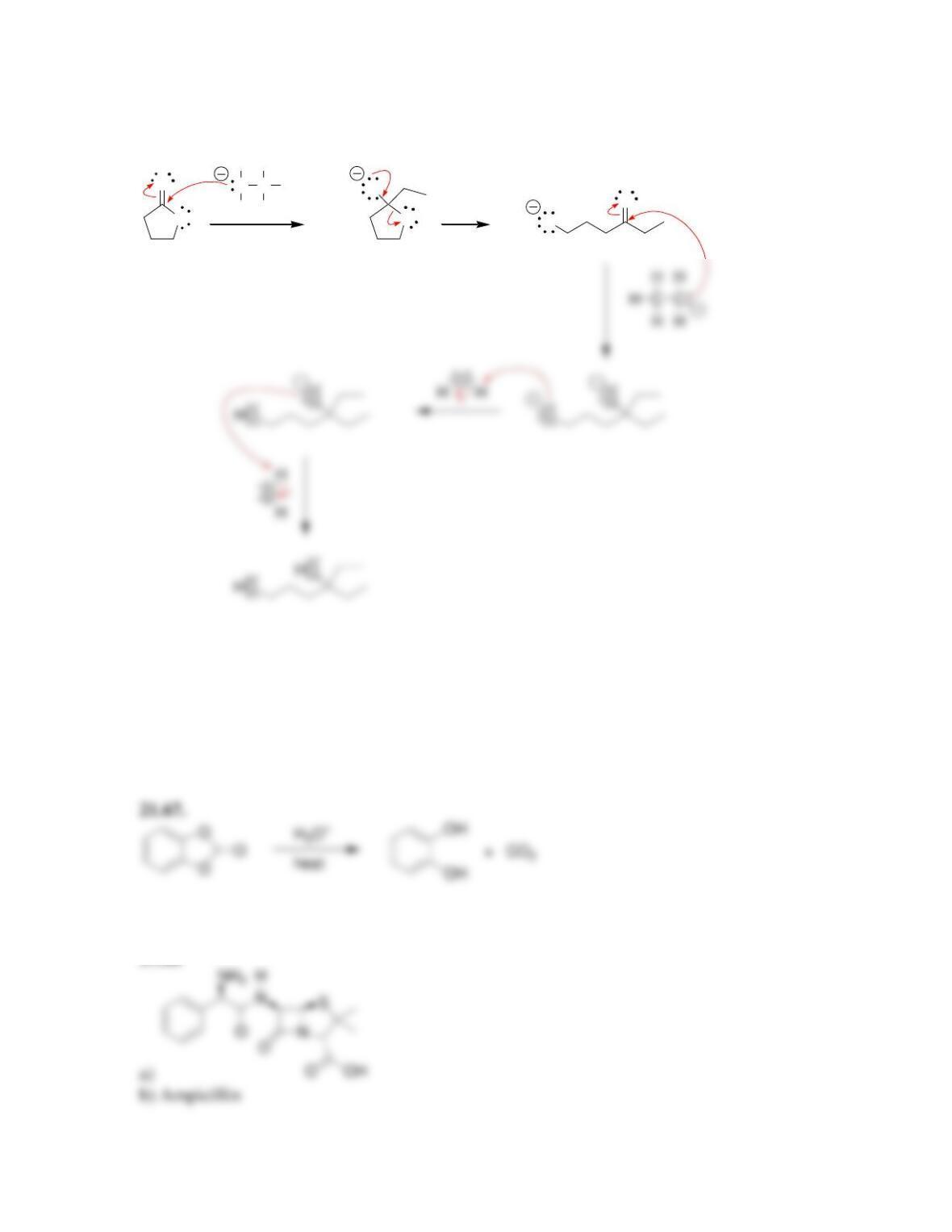
• When drawing a mechanism, avoid formation of ___________ charges in acidic
conditions, and avoid formation of ____________ charges in alkaline conditions.
• In a process called the Fischer esterification, carboxylic acids are converted into
esters when treated with an ____________ in the presence of ________________.
• Esters can be hydrolyzed to yield carboxylic acids by treatment with either
aqueous base or aqueous _______. Hydrolysis under basic conditions is also
called ________________.