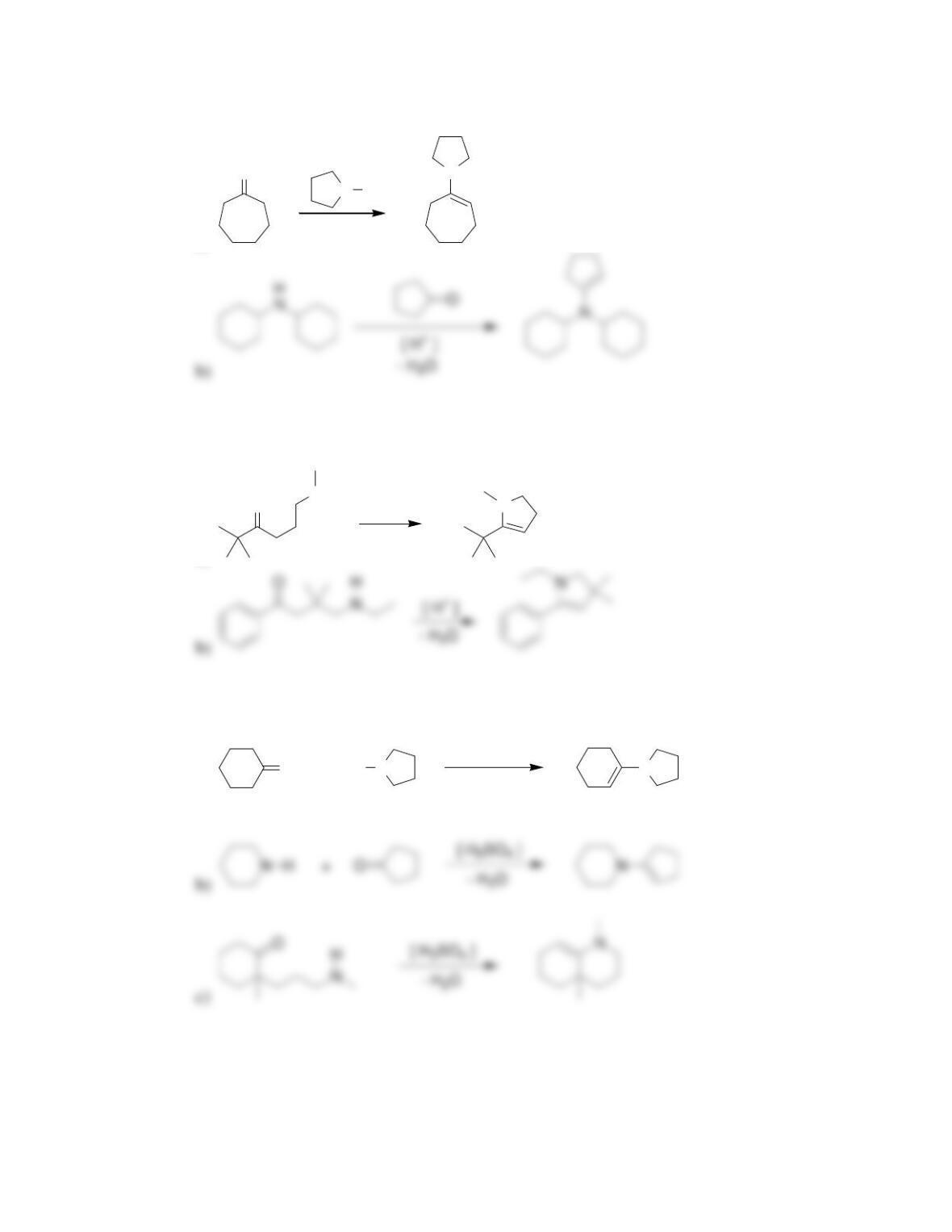
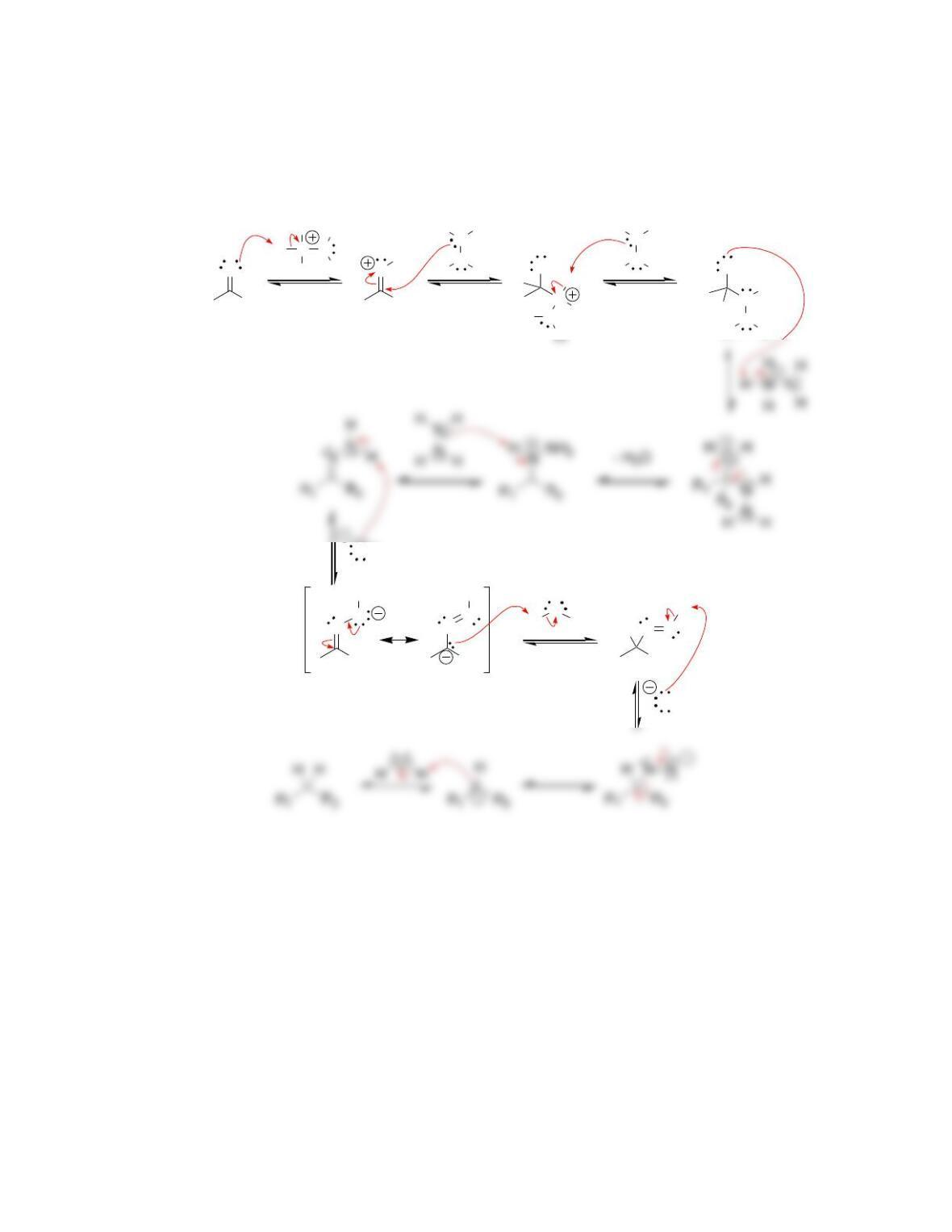
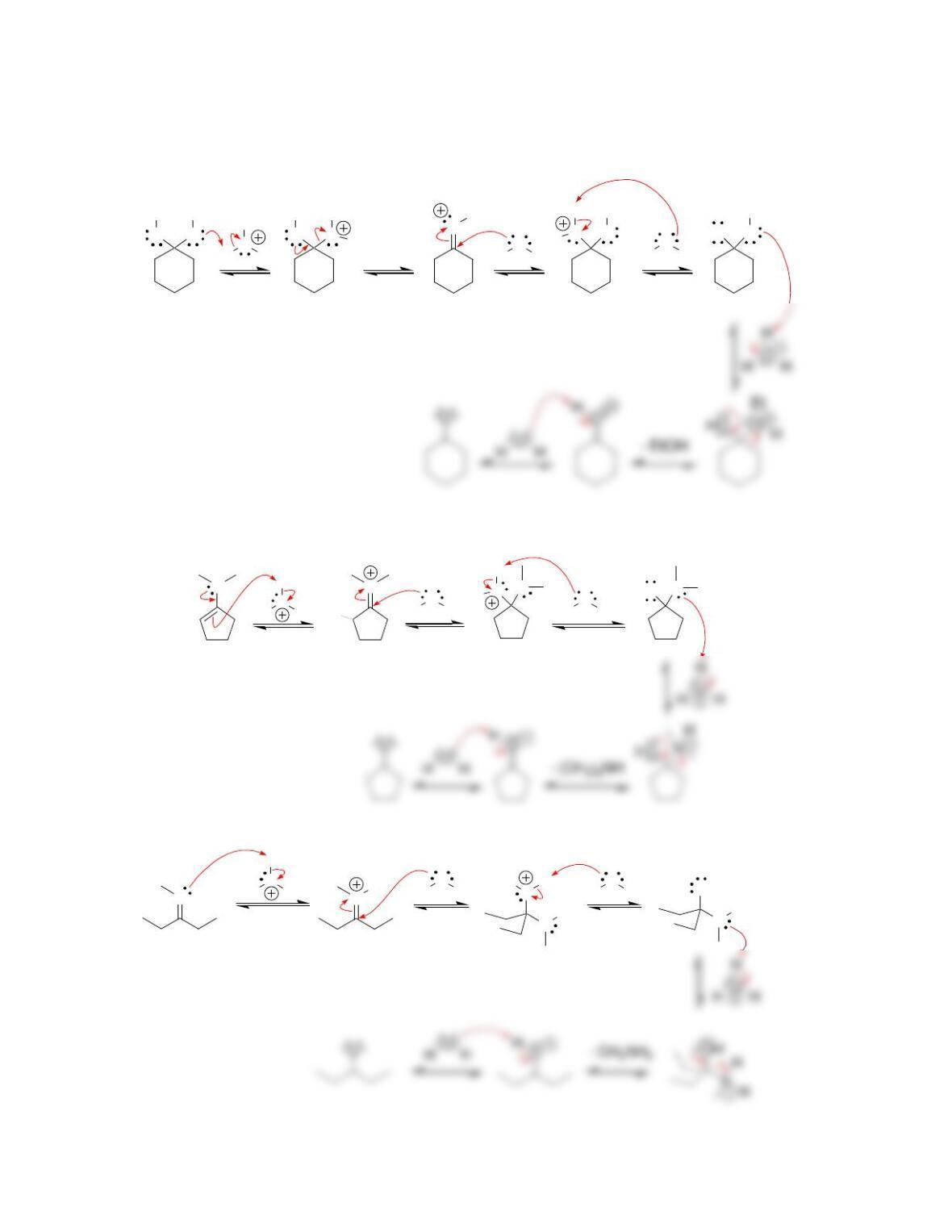
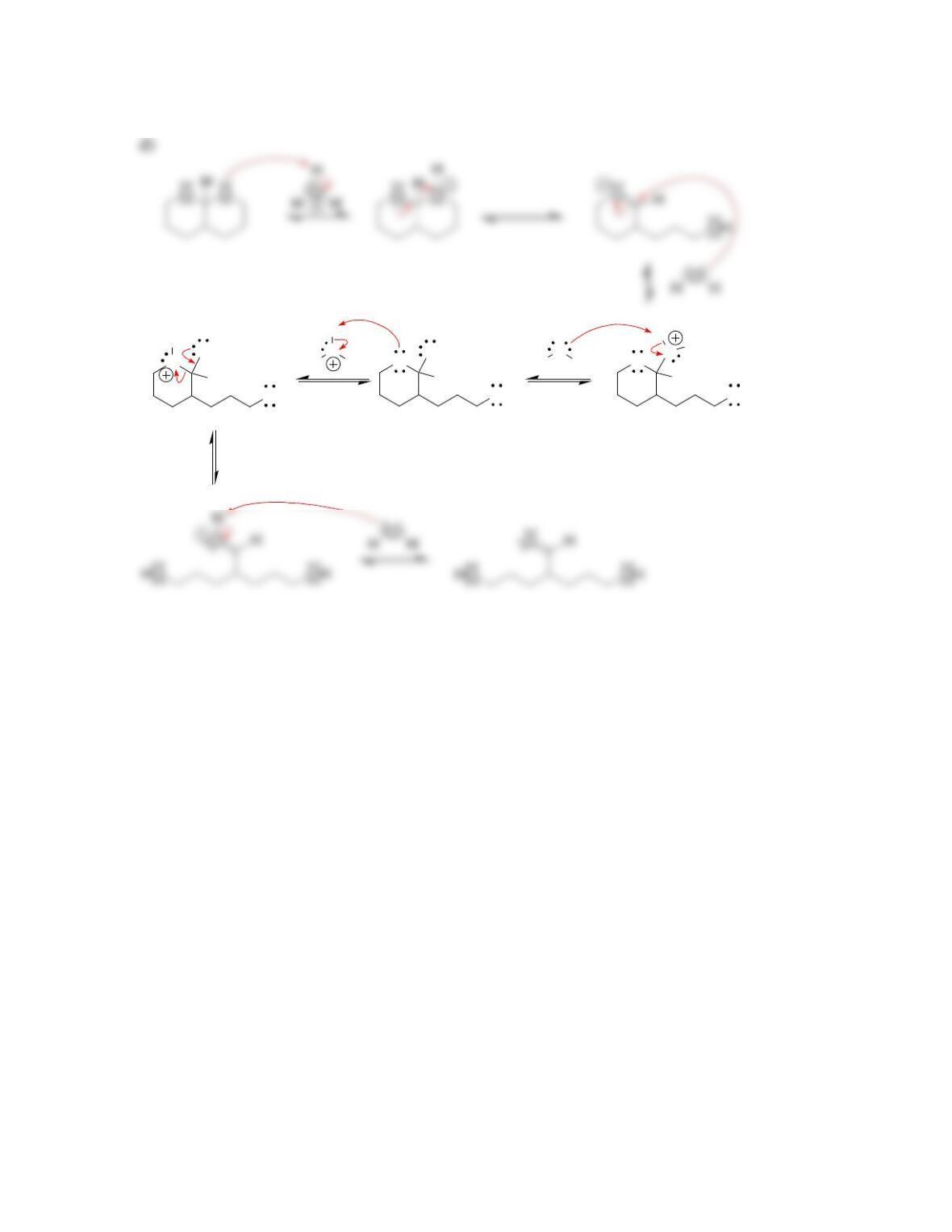
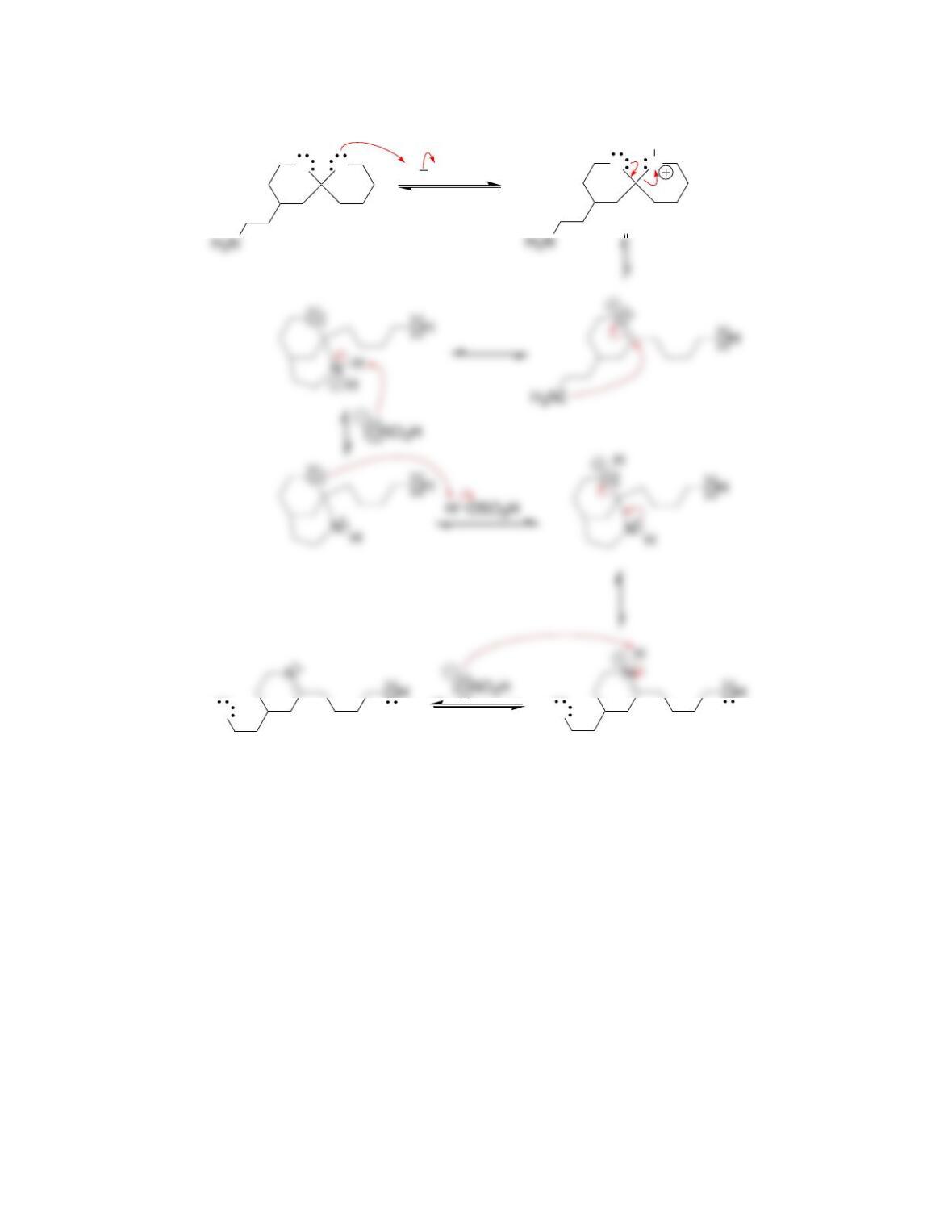
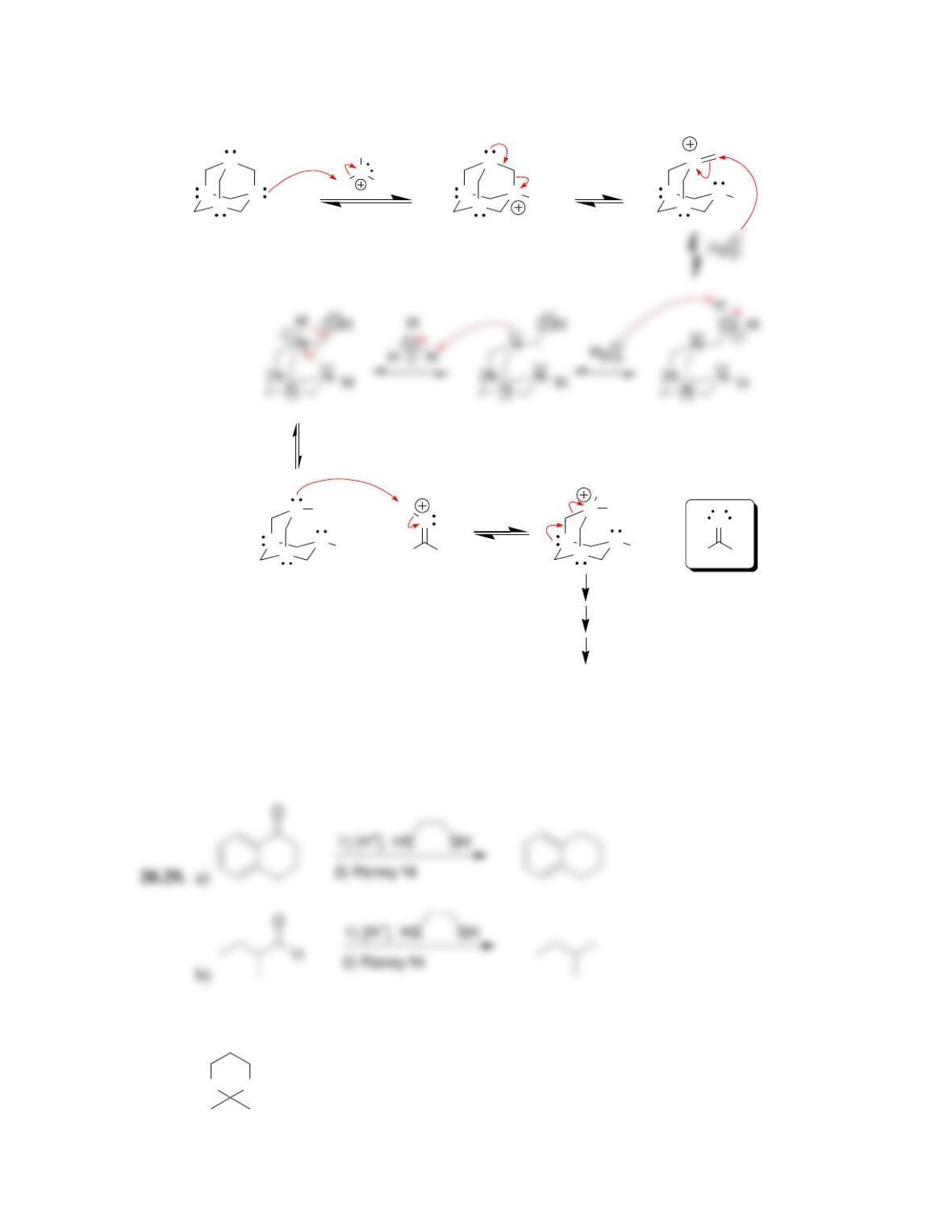
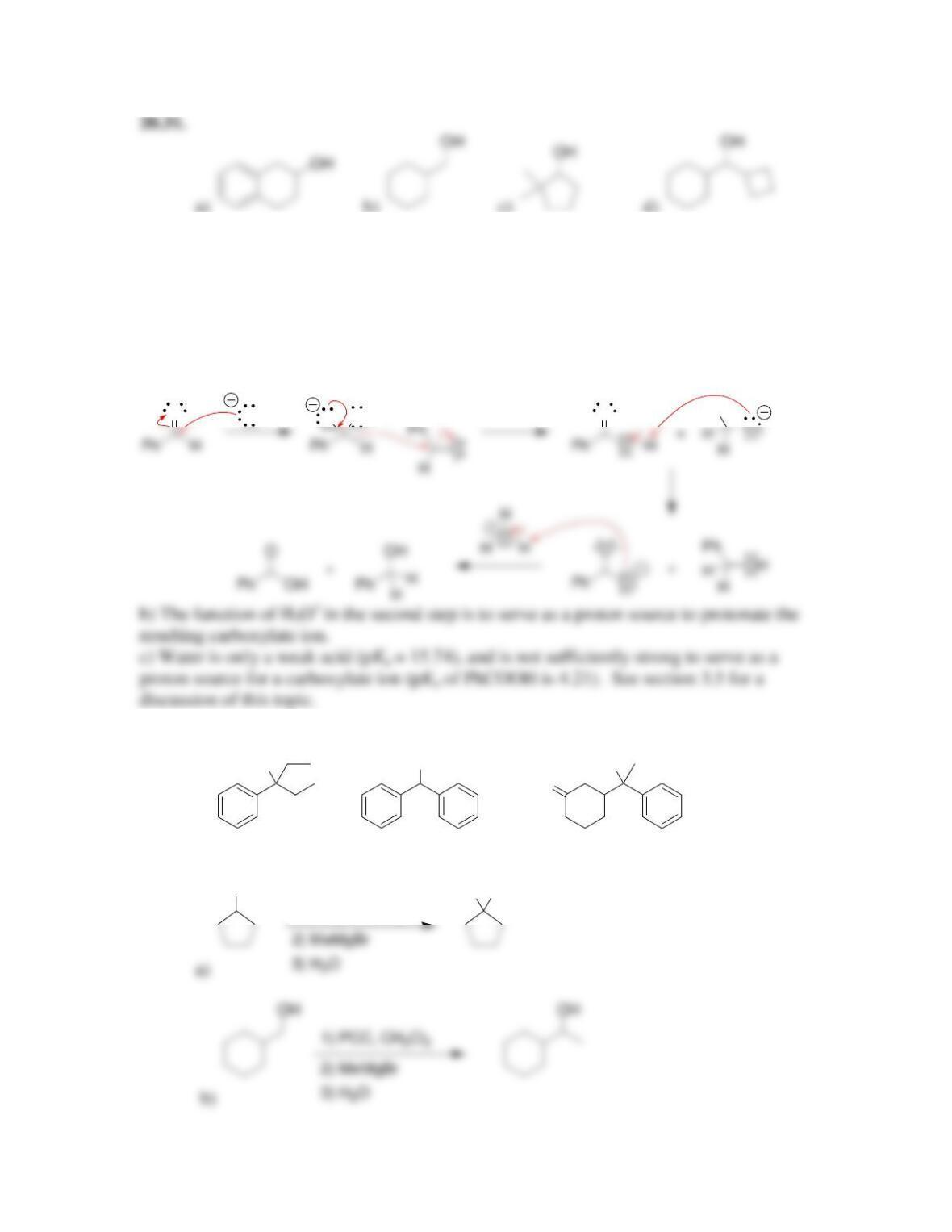
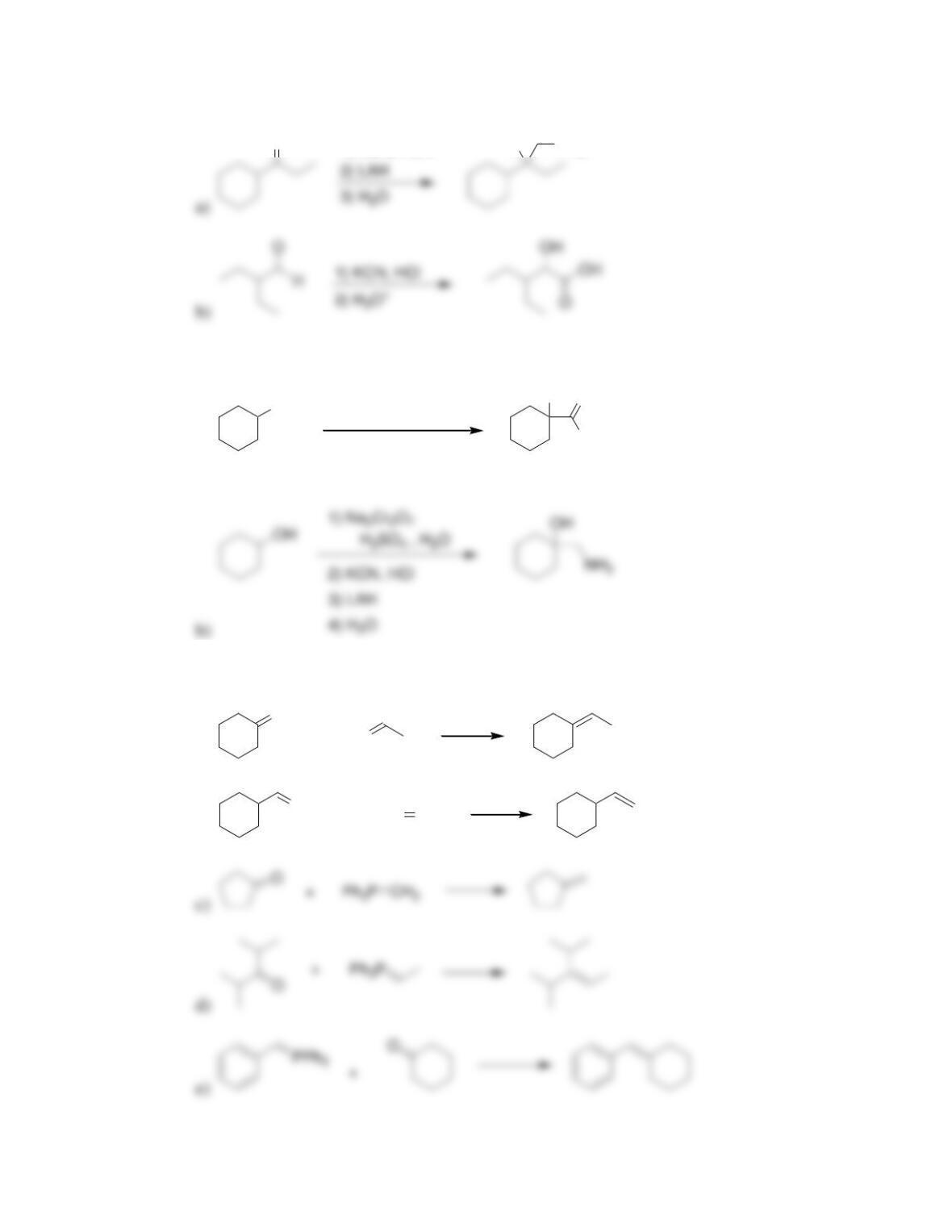
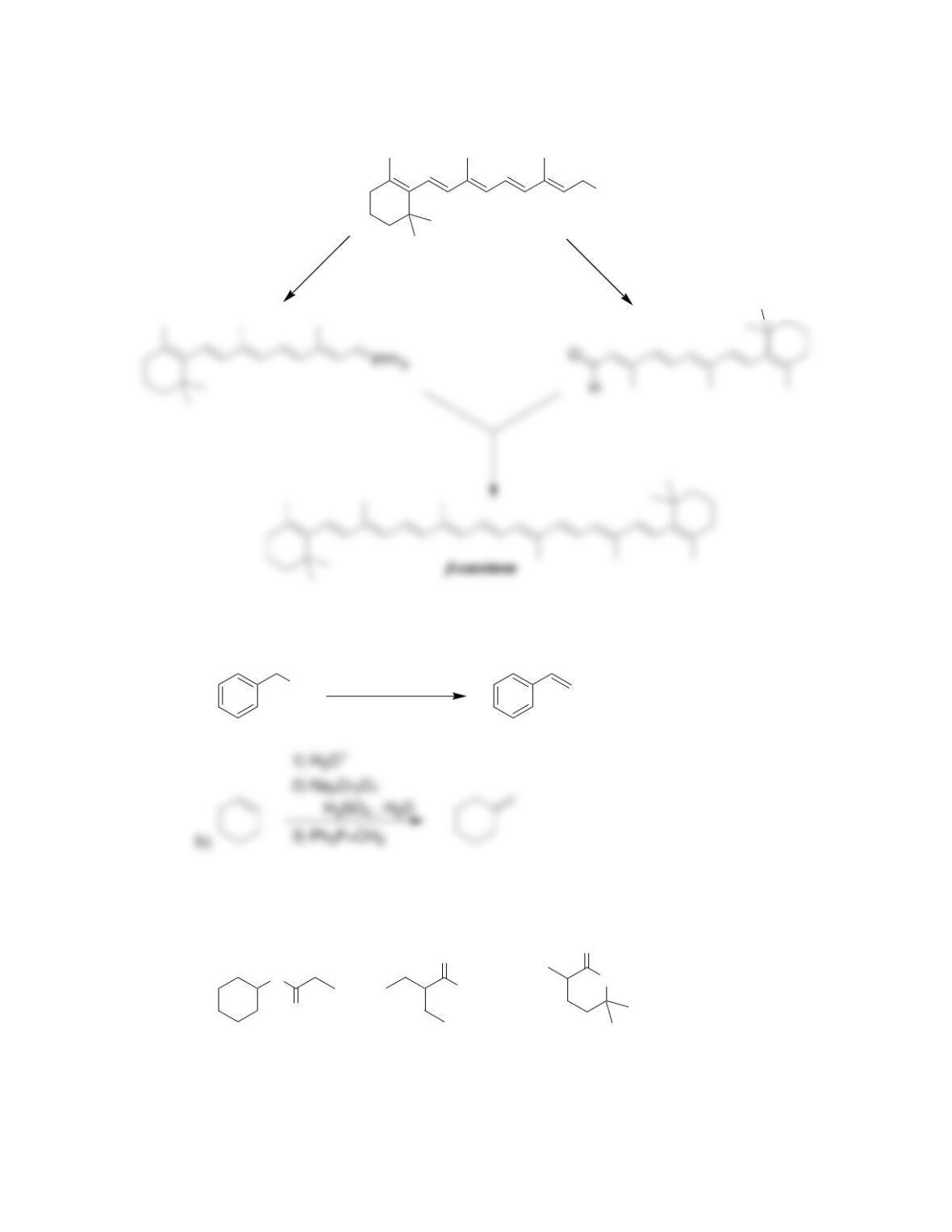
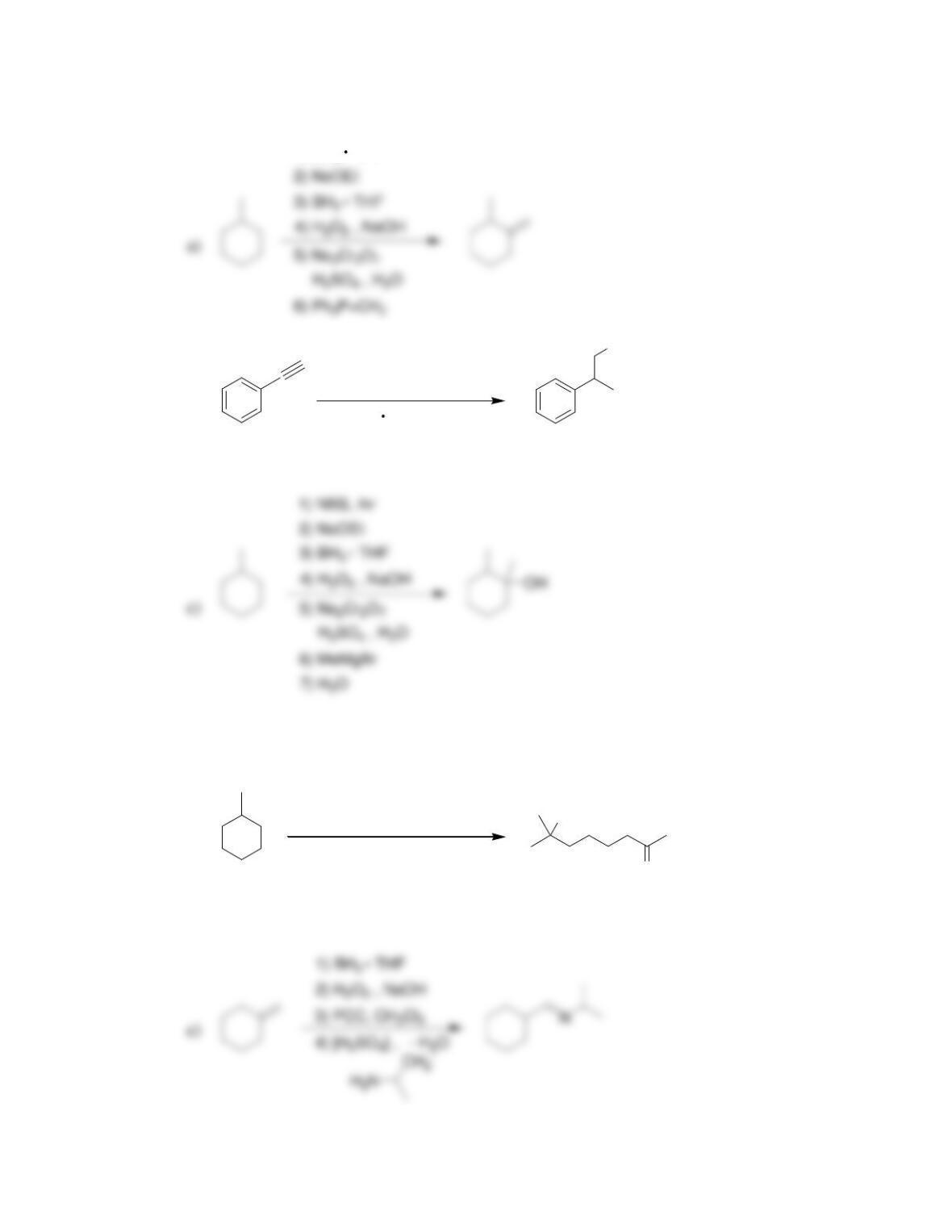
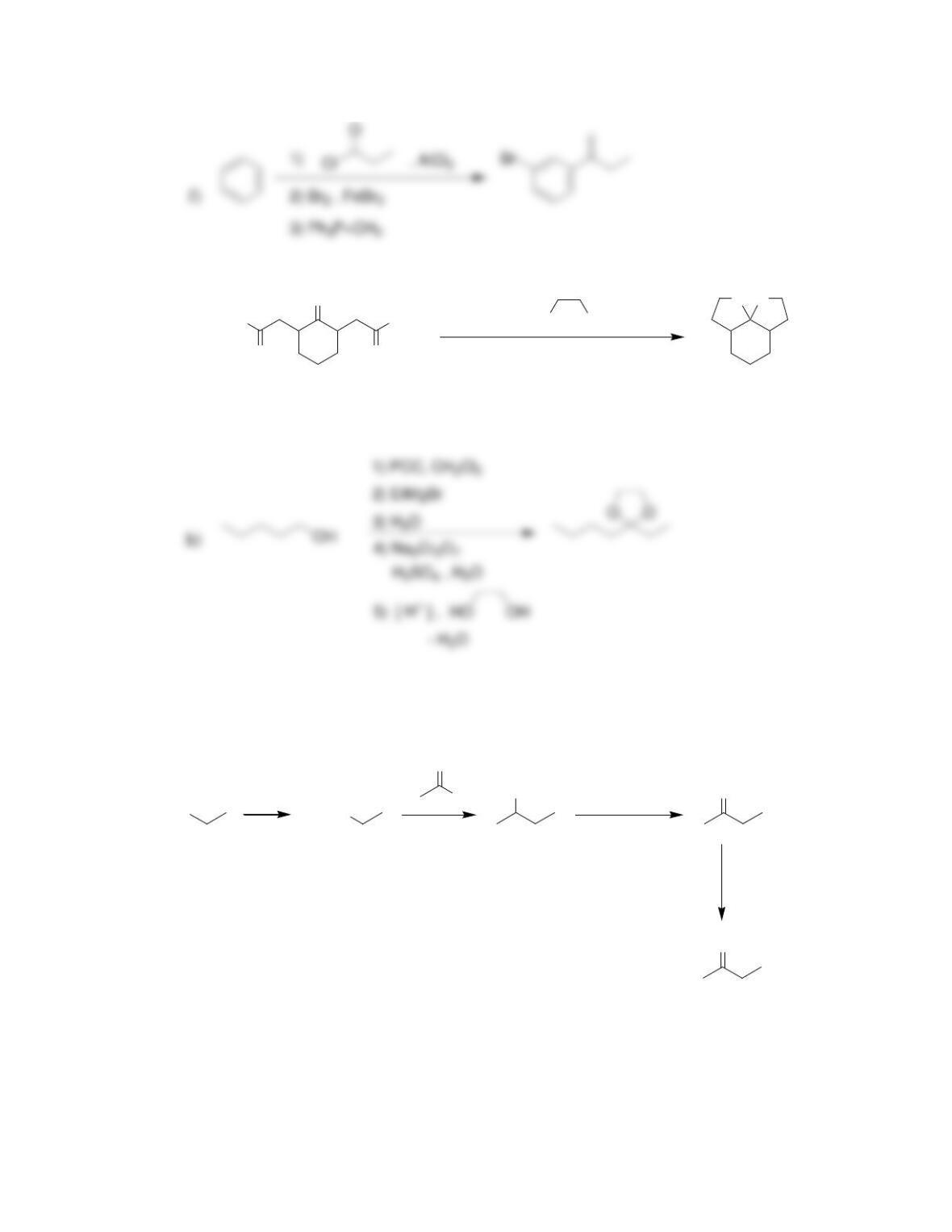
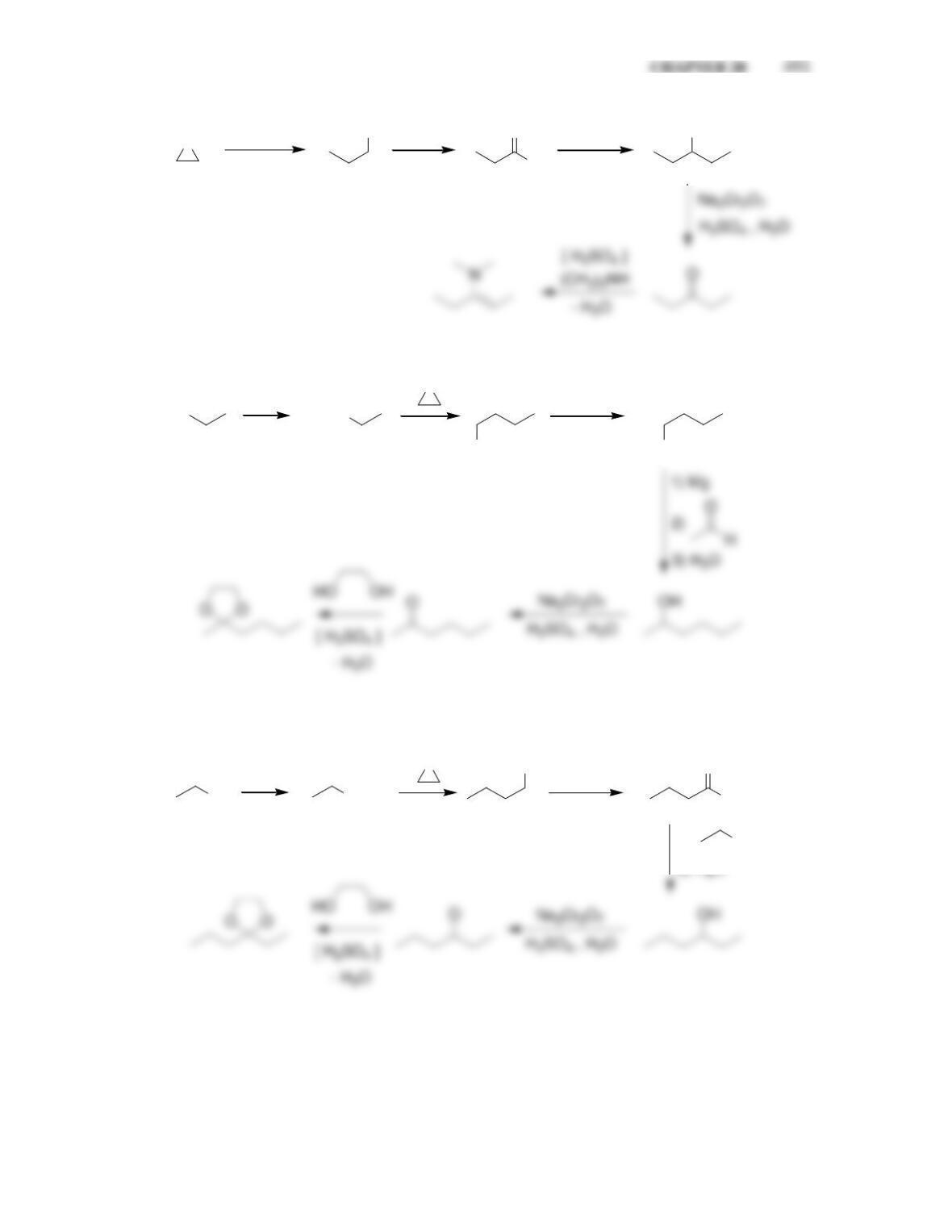
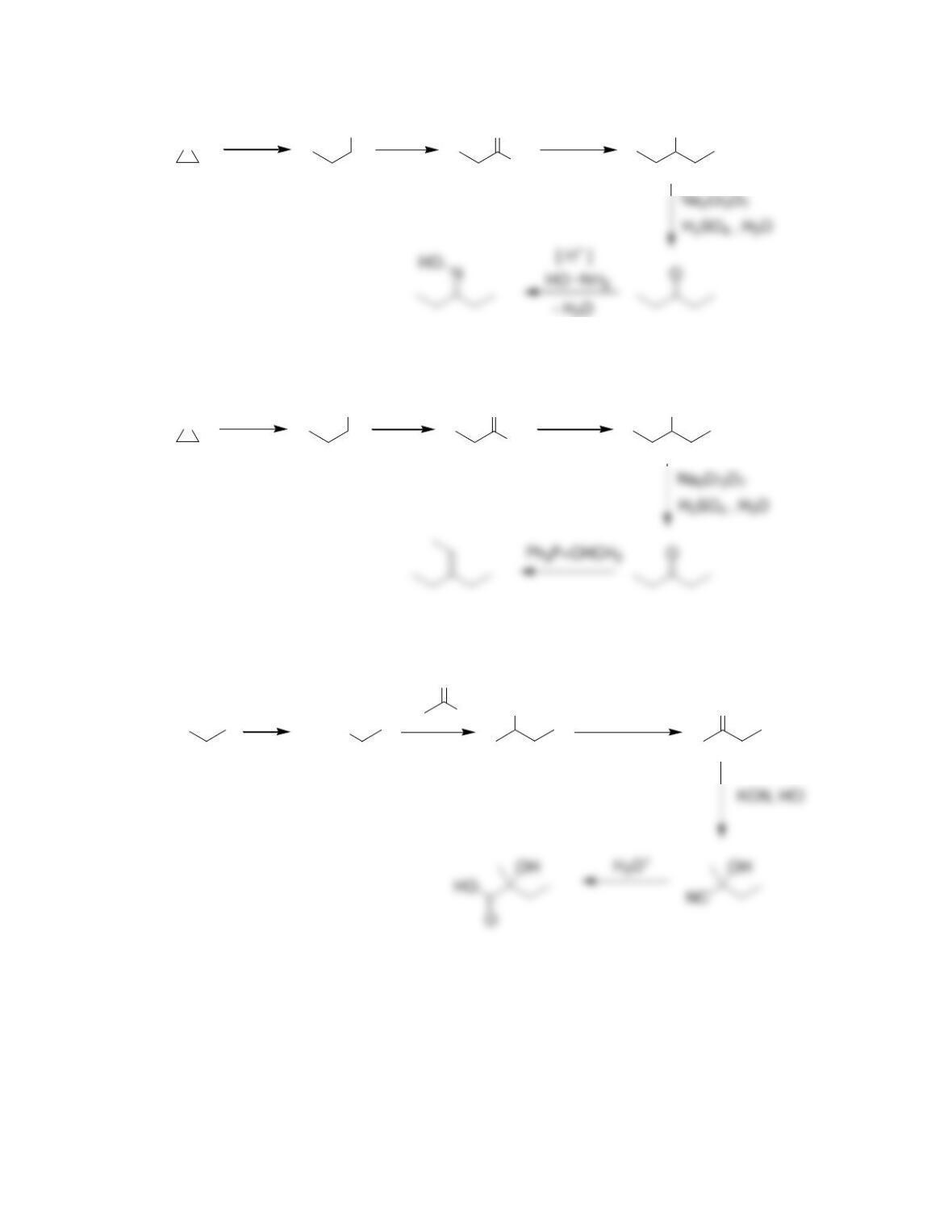
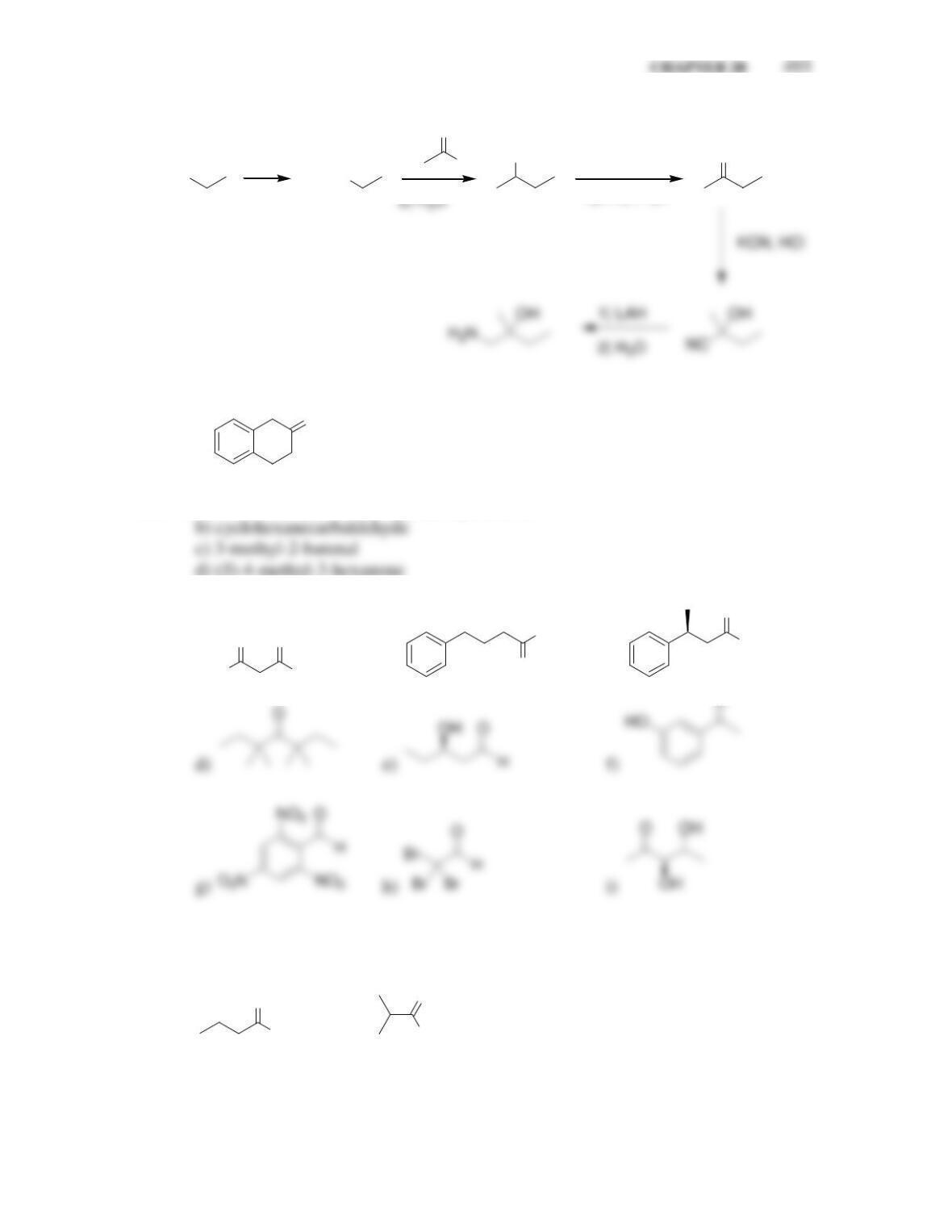
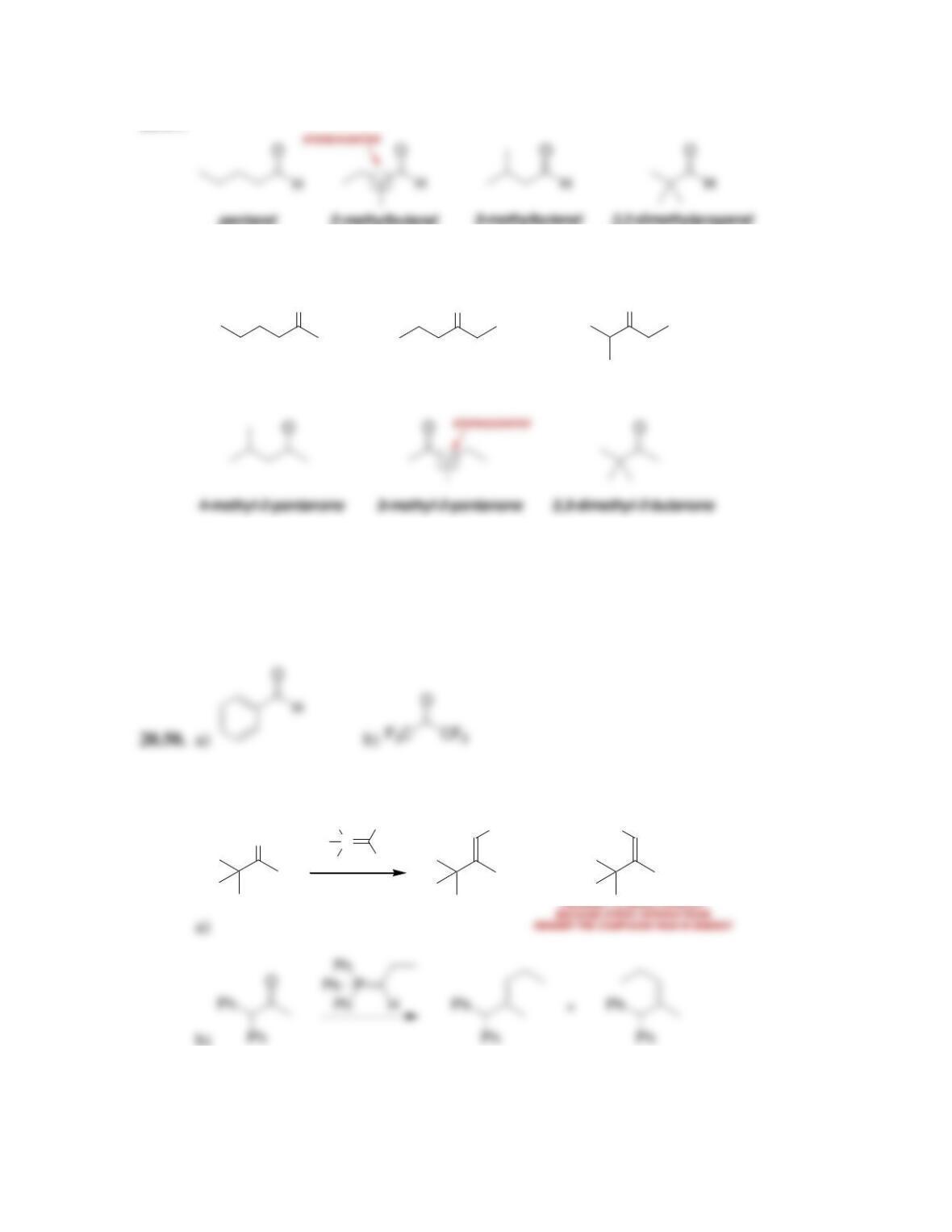
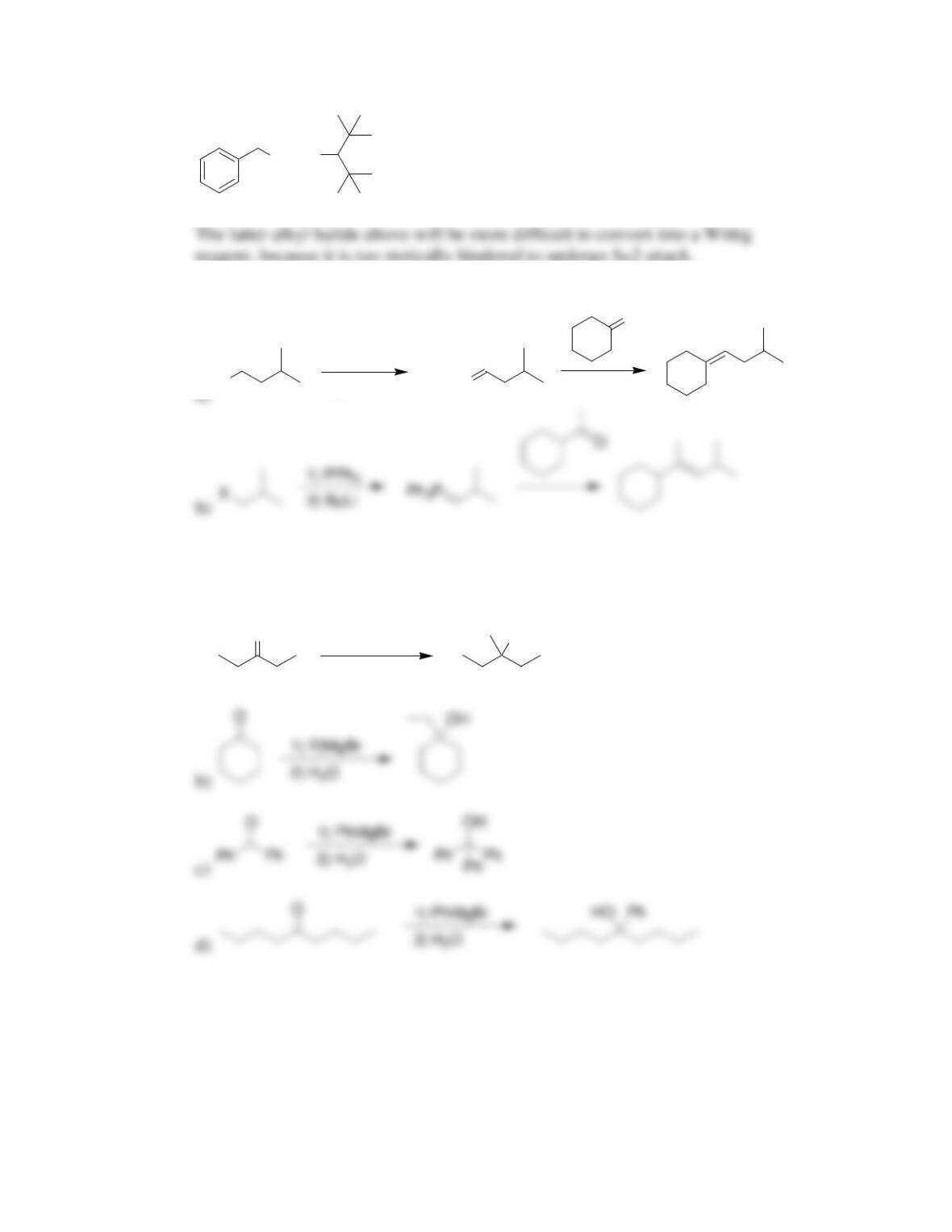
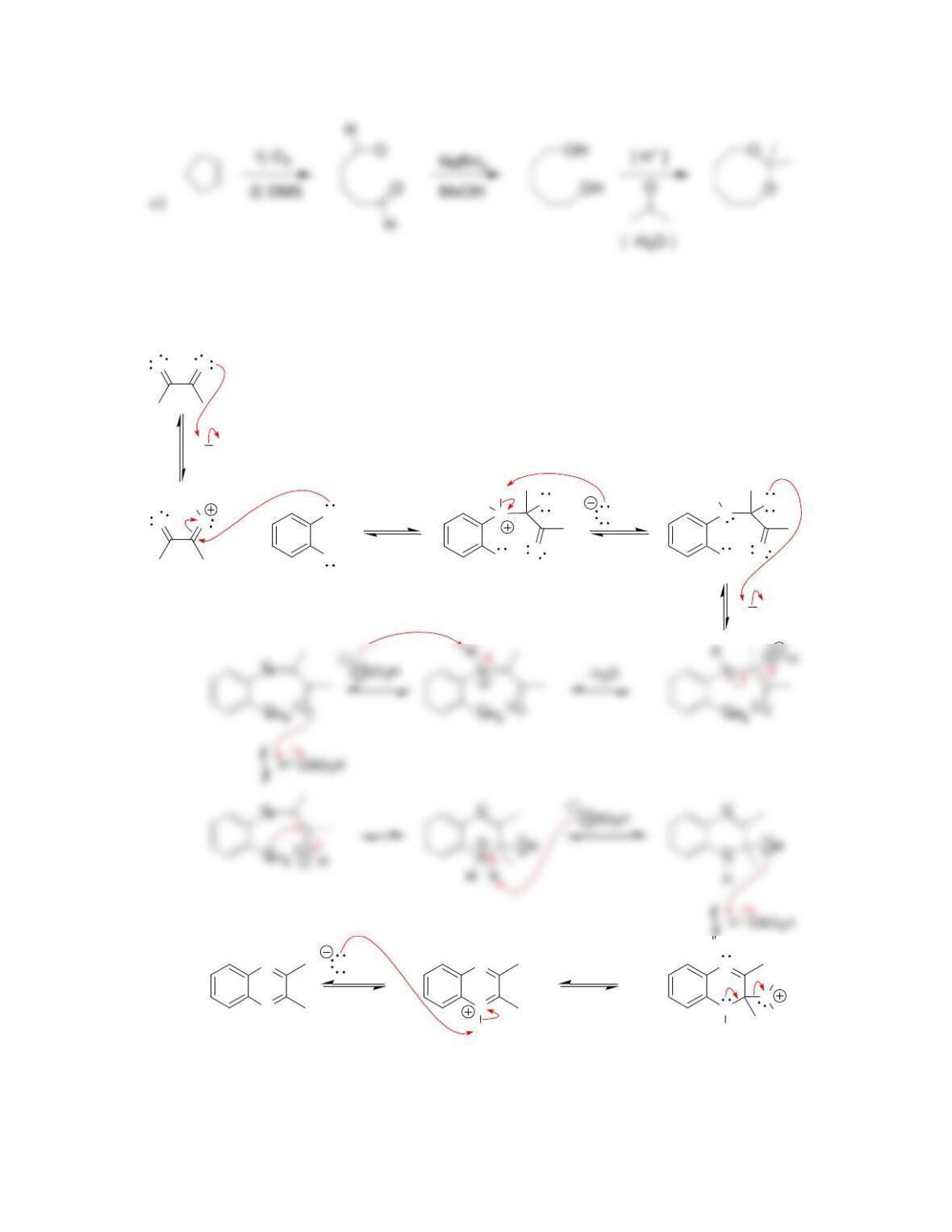
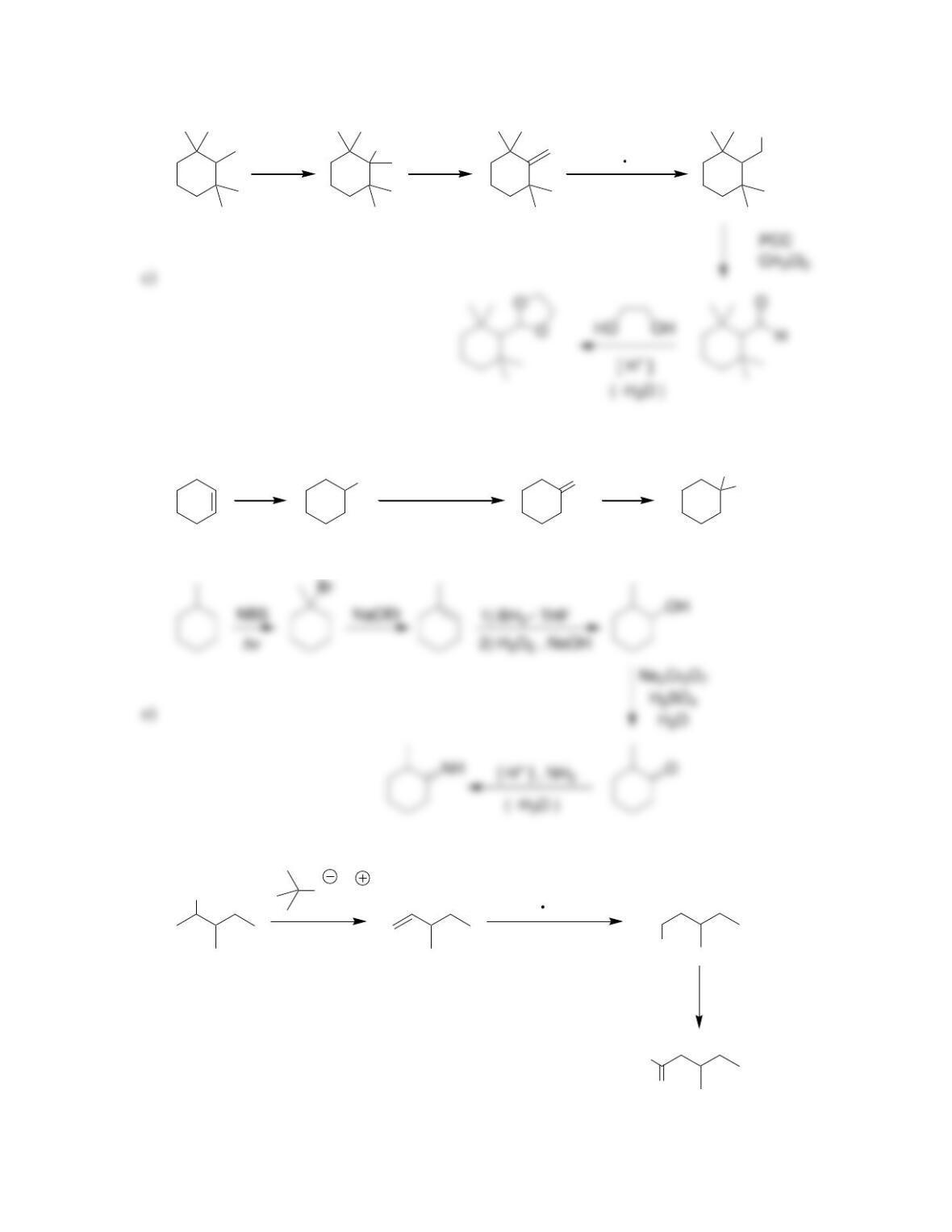
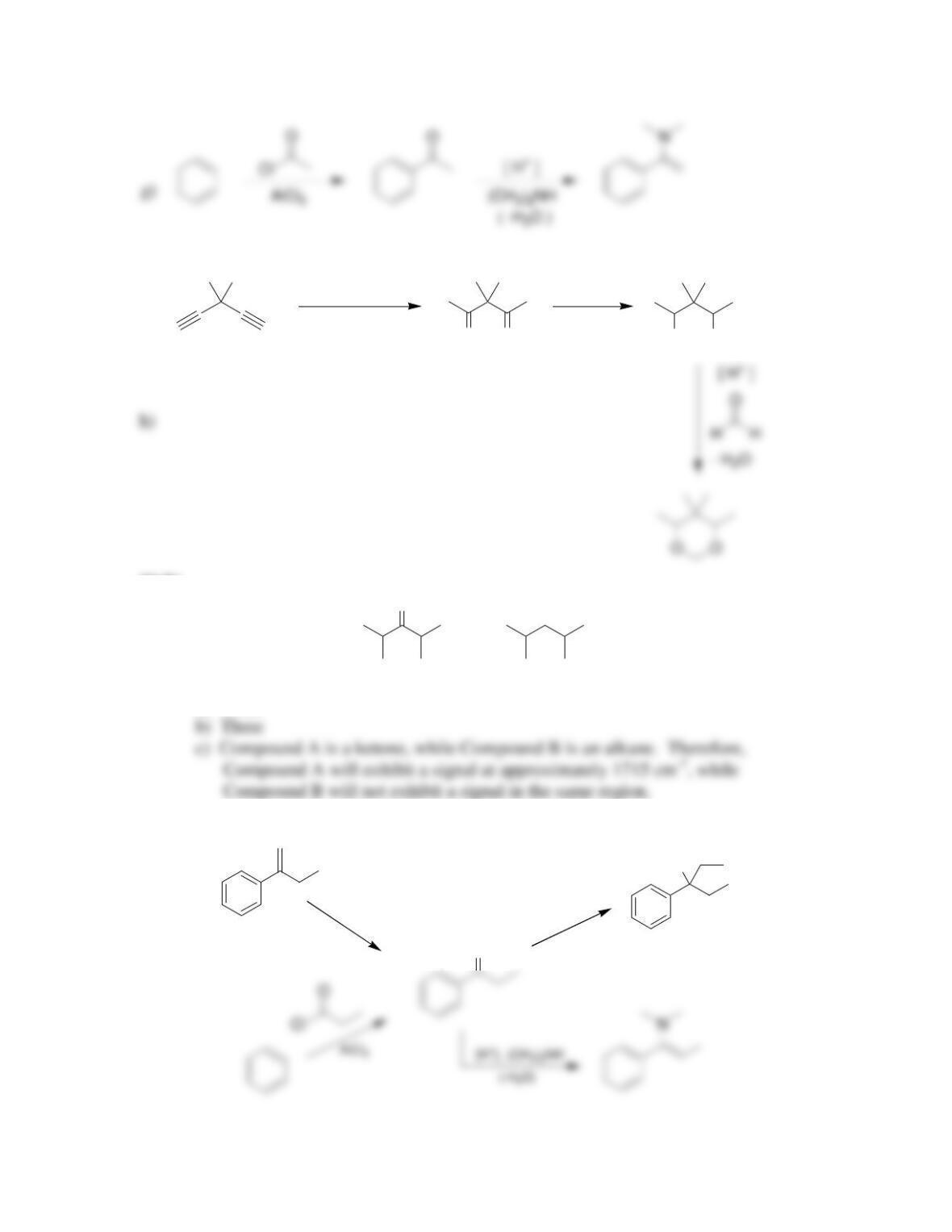
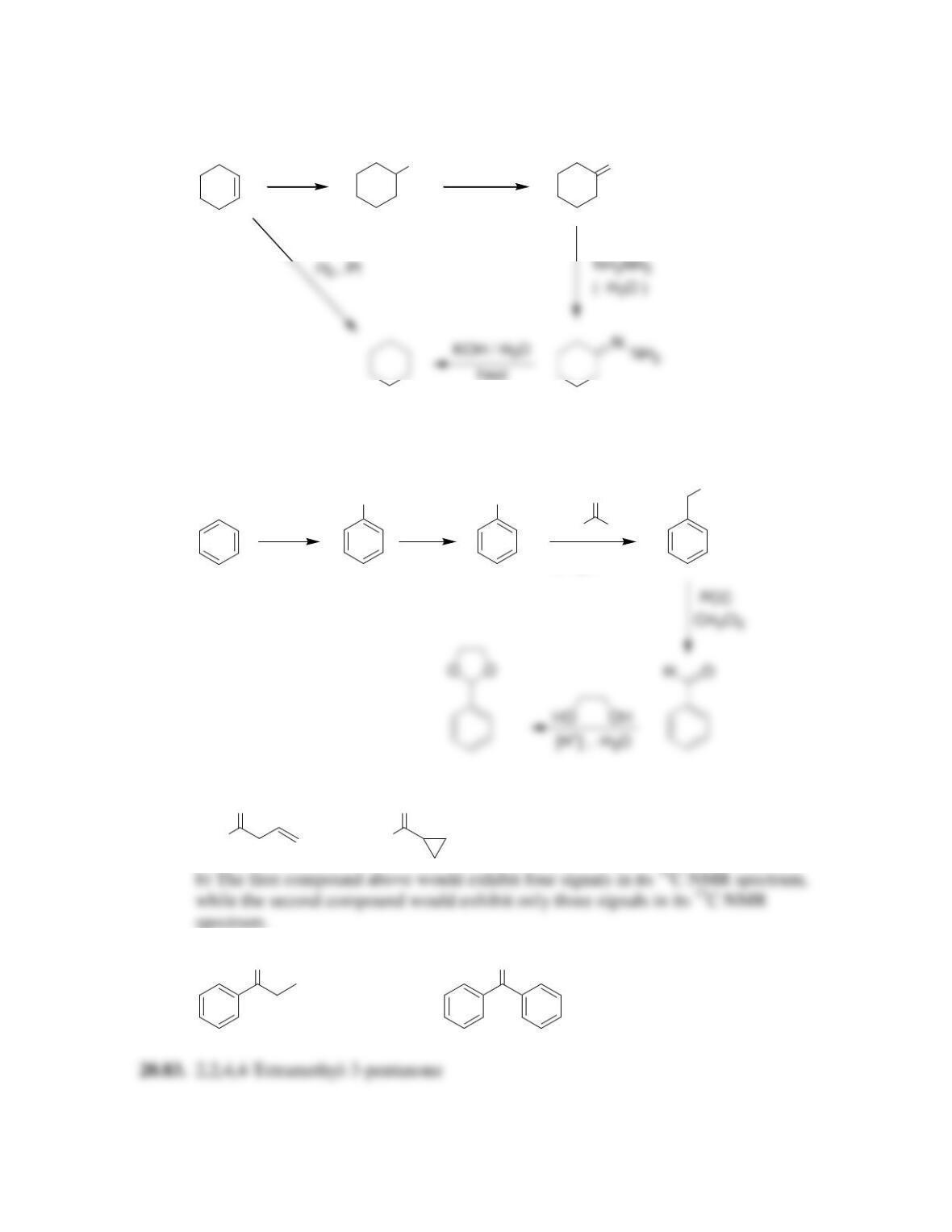
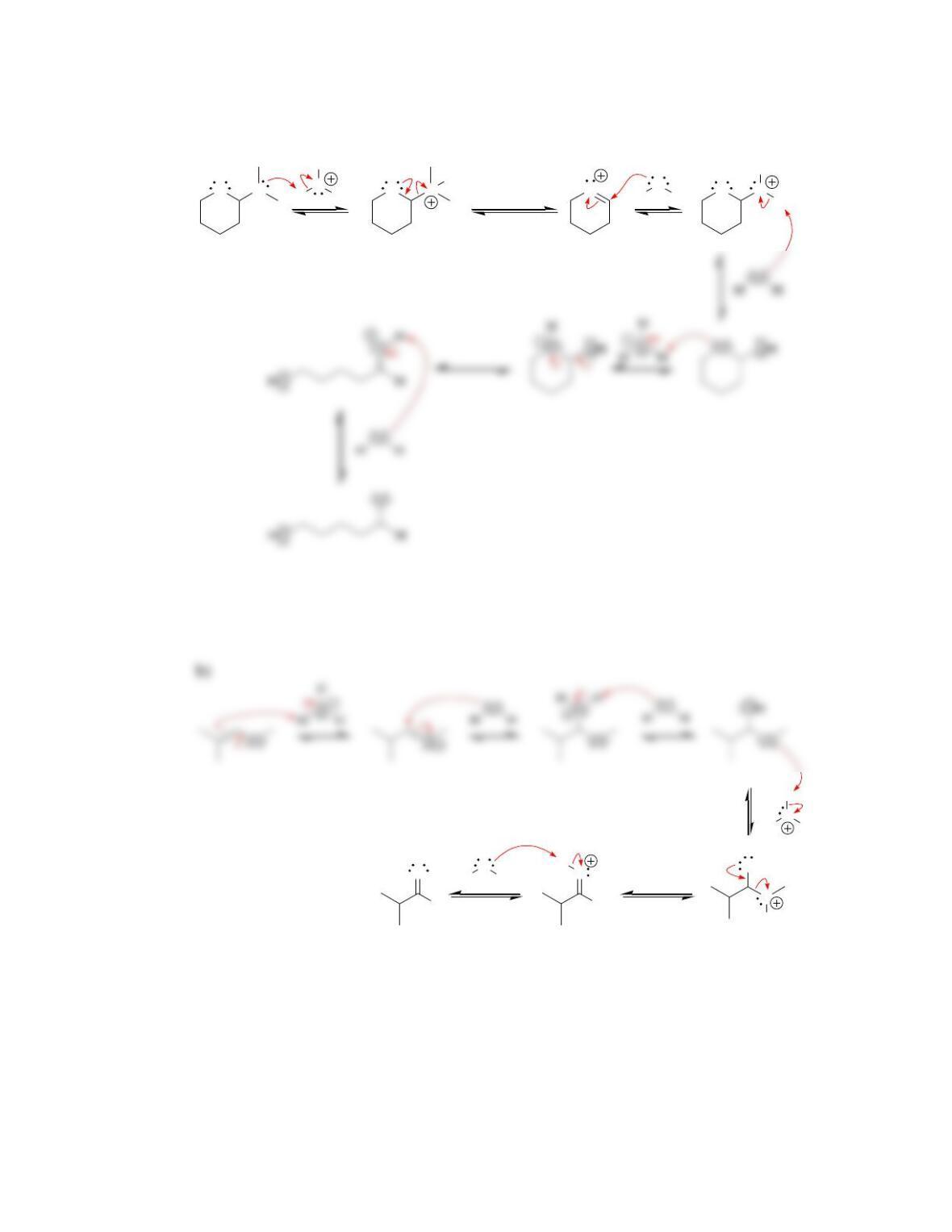
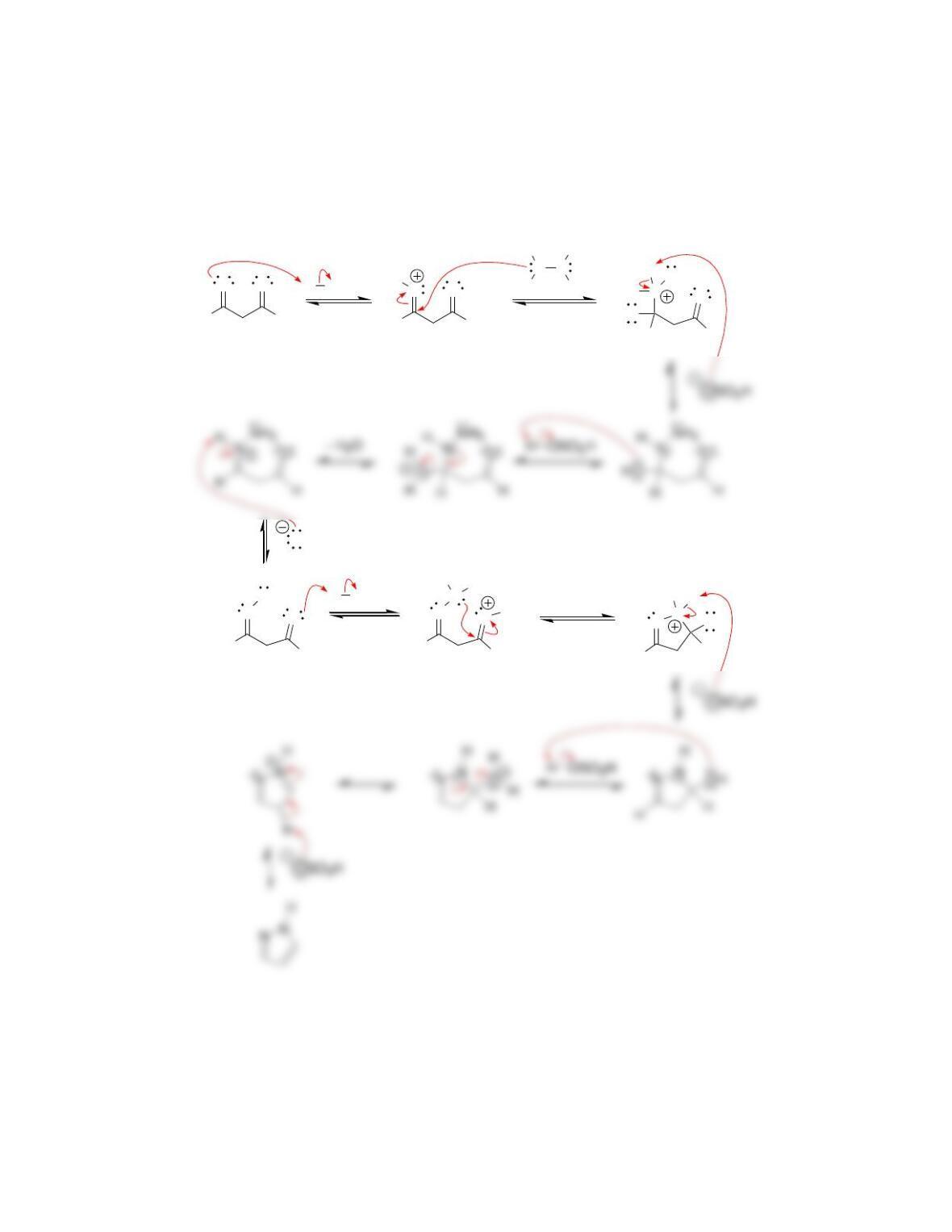
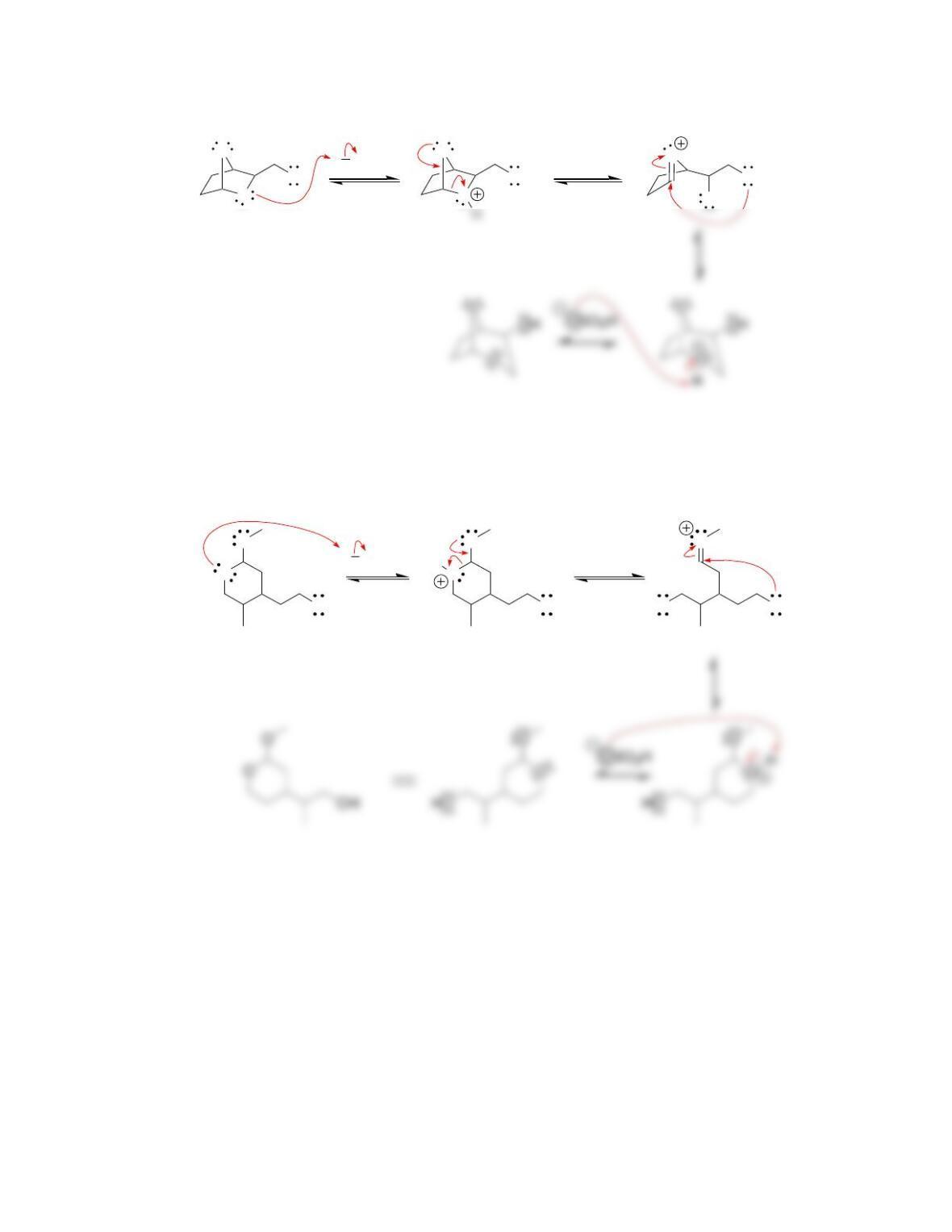
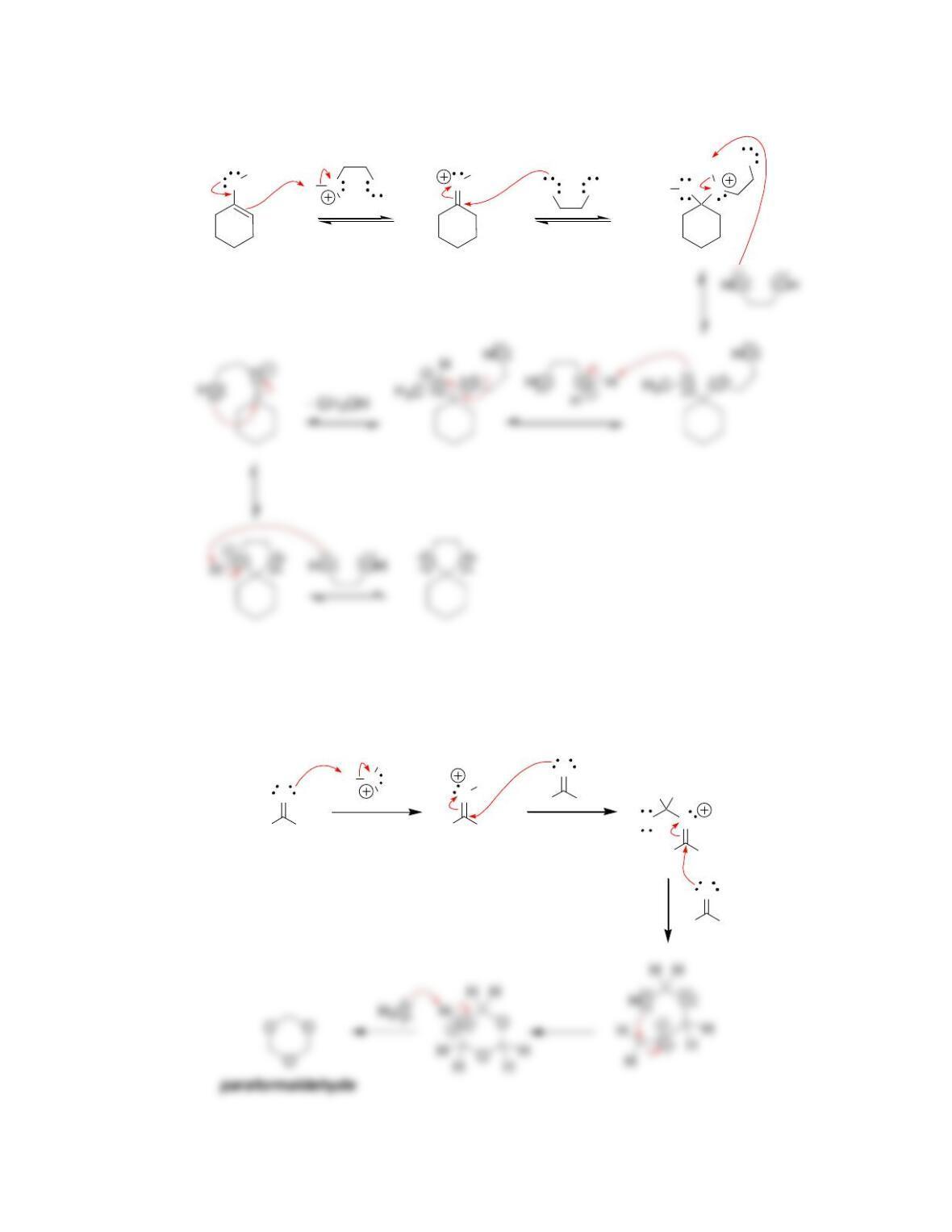
Chapter 20
Ketones and Aldehydes
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 20. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• The suffix “_____” indicates an aldehydic group, and the suffix “_____” is used
for ketones.
• The position of equilibrium is dependent on the ability of the nucleophile to
function as a _______________.
• In acidic conditions, an aldehyde or ketone will react with two molecules of
alcohol to form an __________.
be ____________ or should bear one ___________ charge.
• _________________ of acetals, imines, and enamines under acidic conditions
produces ketones or aldehydes.
• In acidic conditions, an aldehyde or ketone will react with two equivalents of a
thiol to form a ______________.
alcohols, accompanied by the formation of a new ___________ bond.