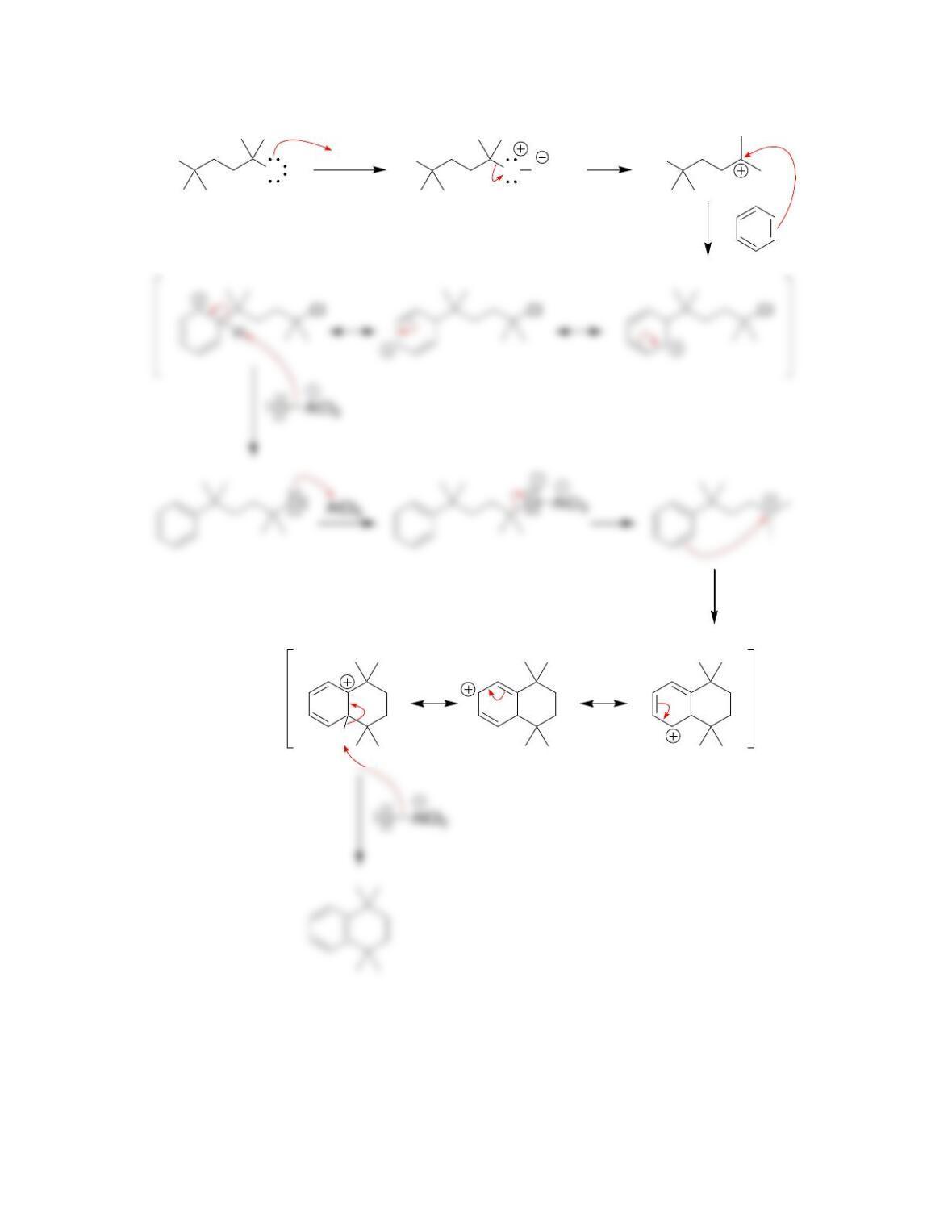
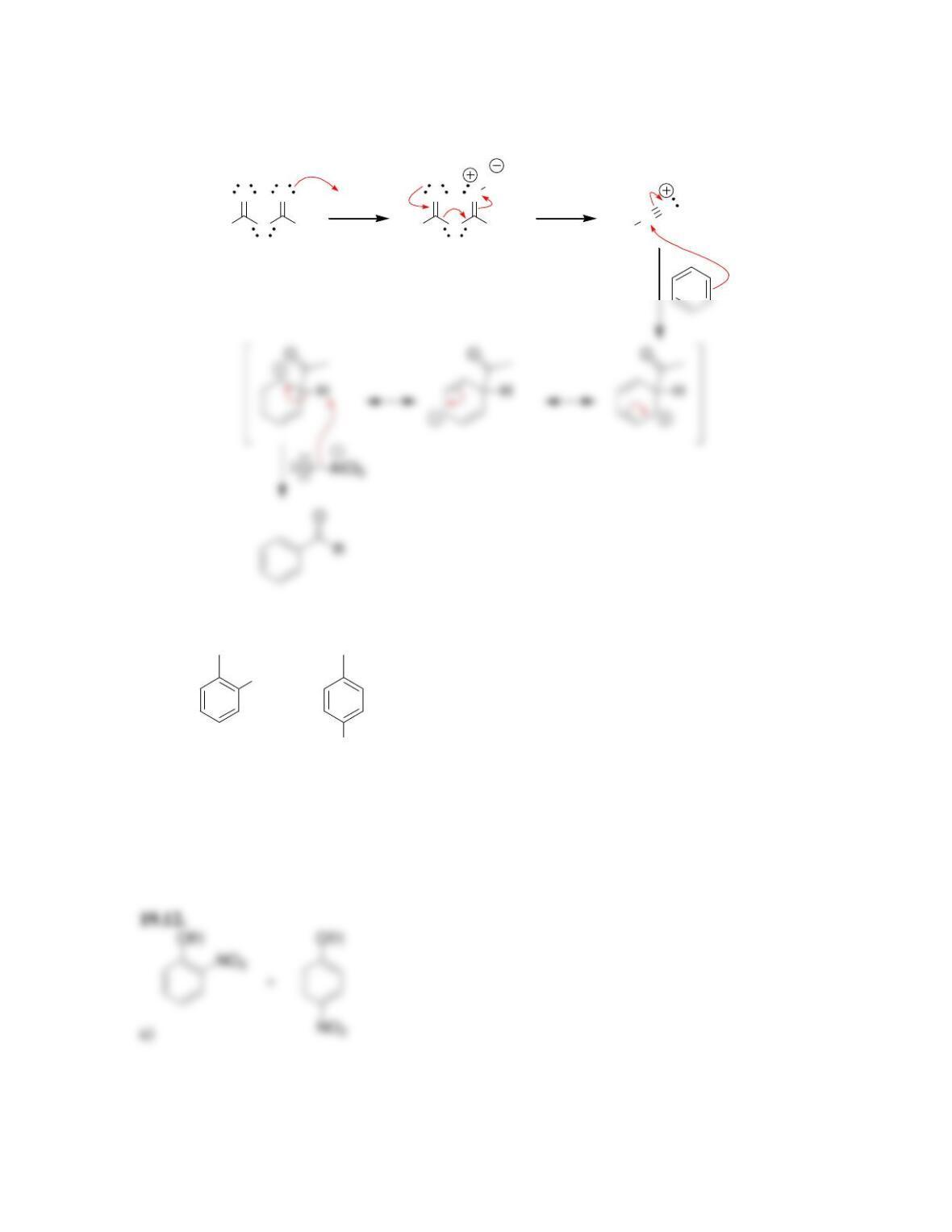
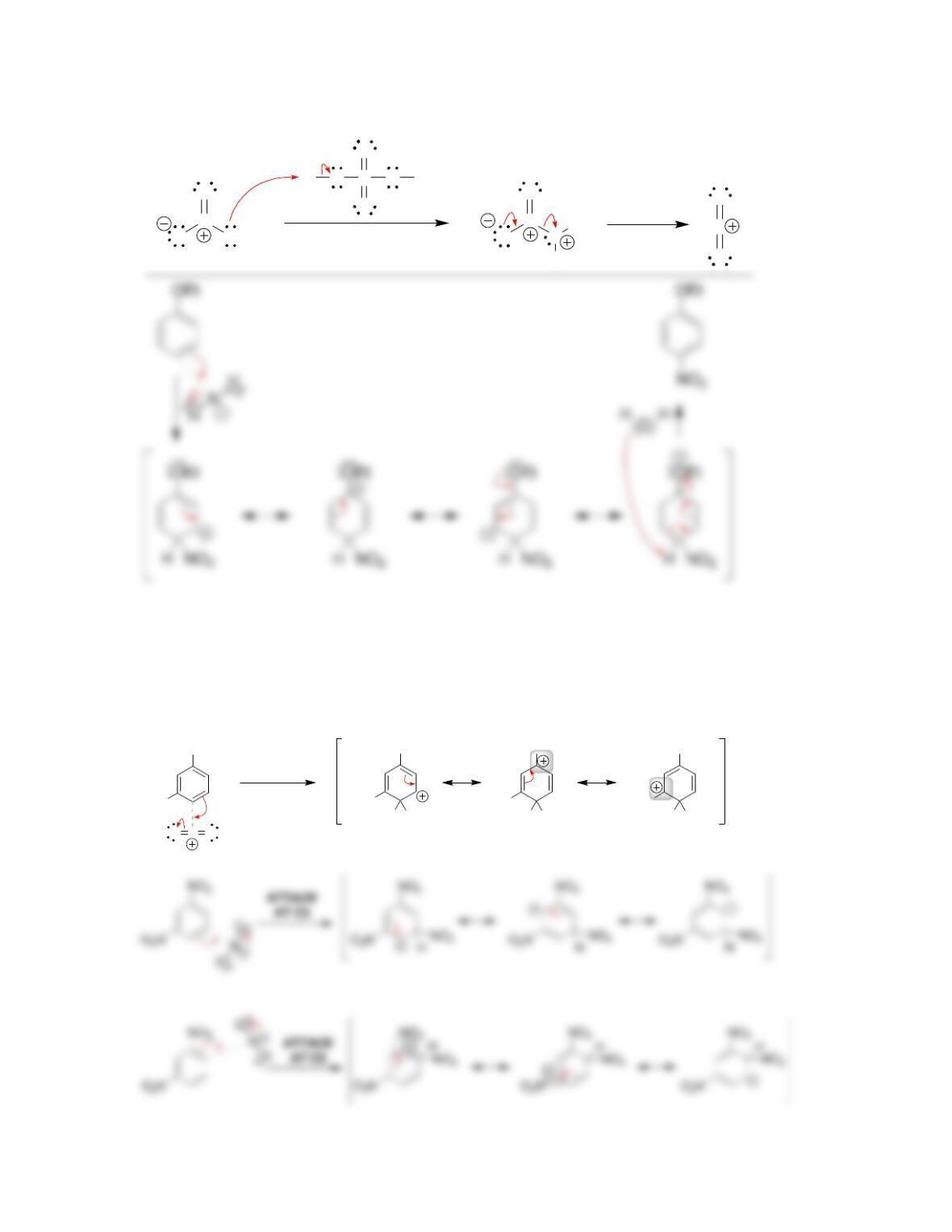
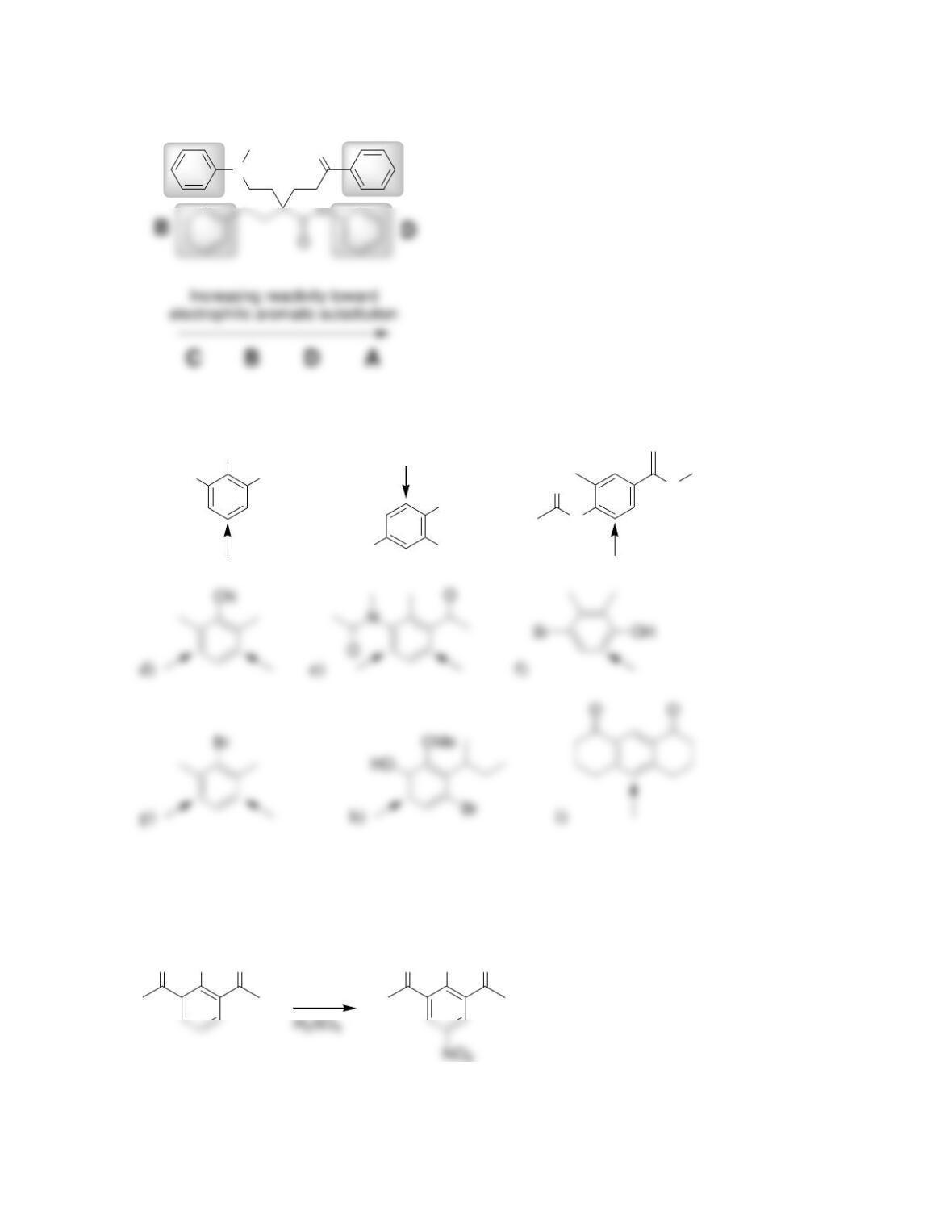
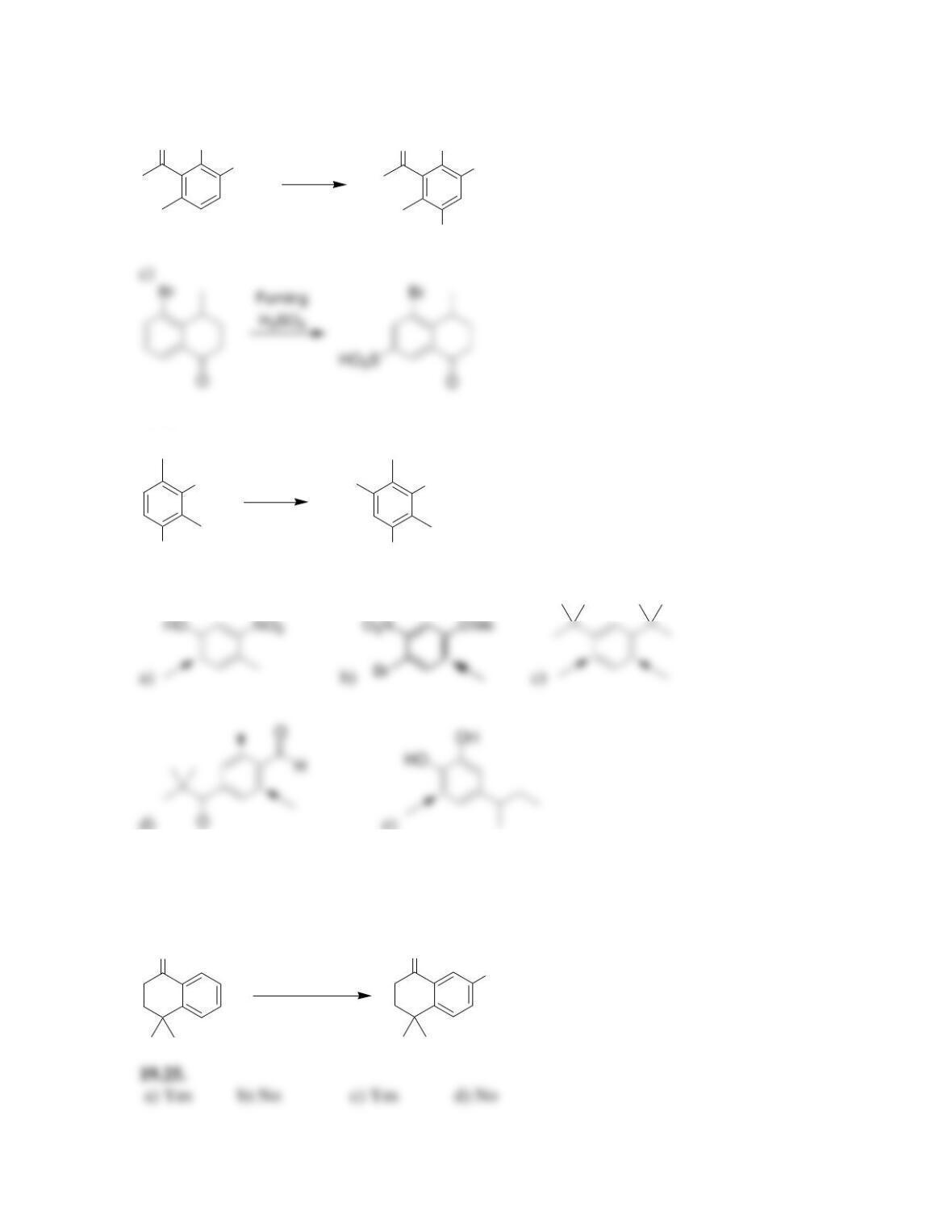
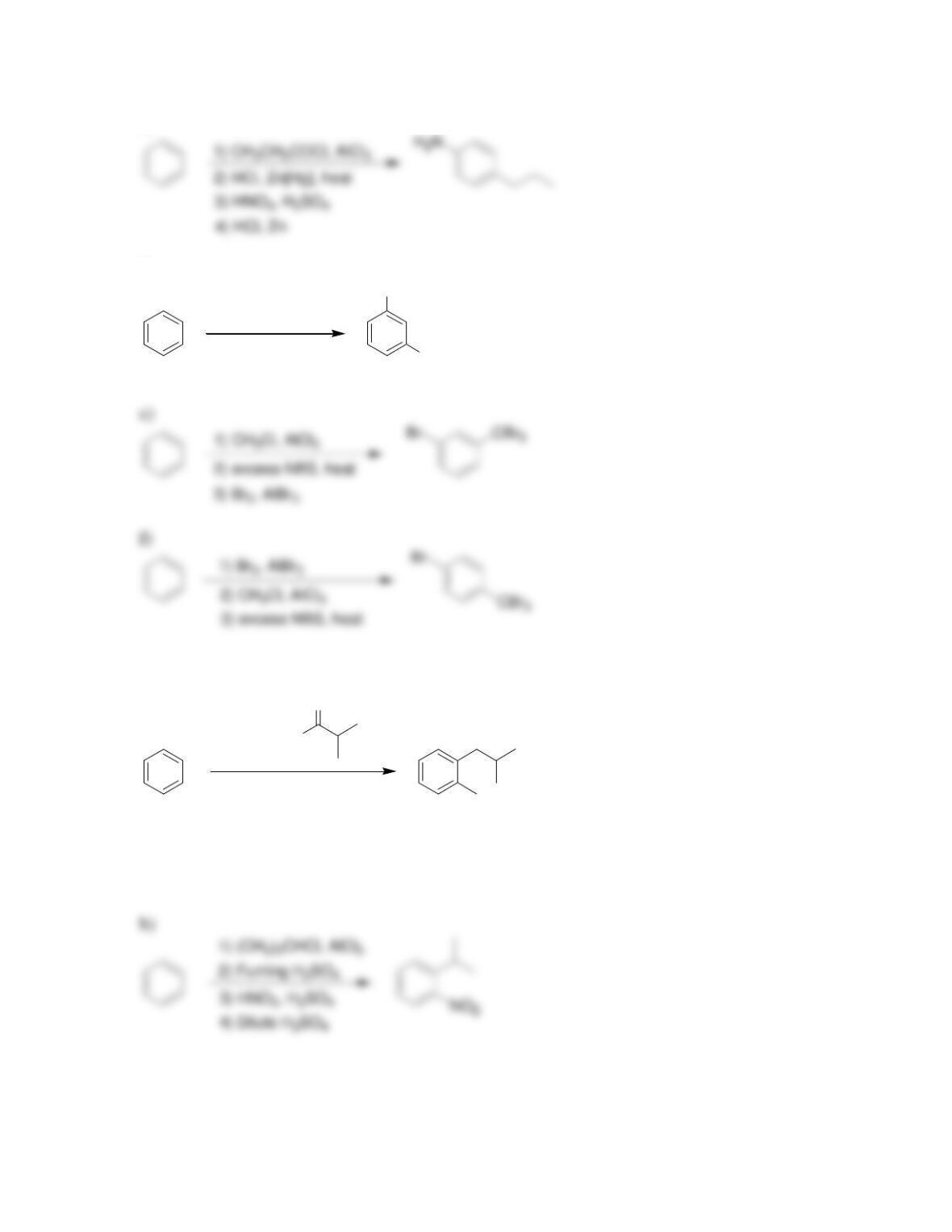
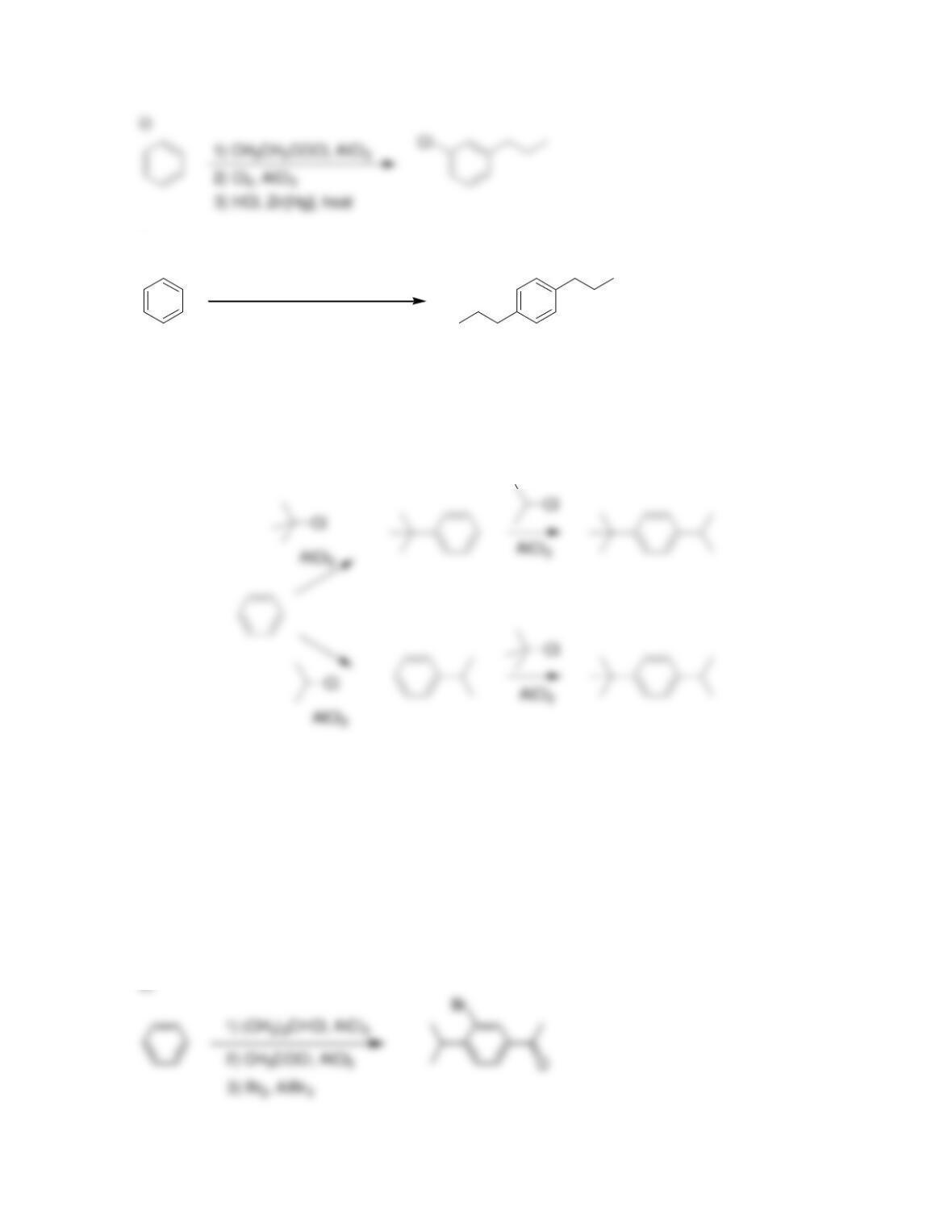
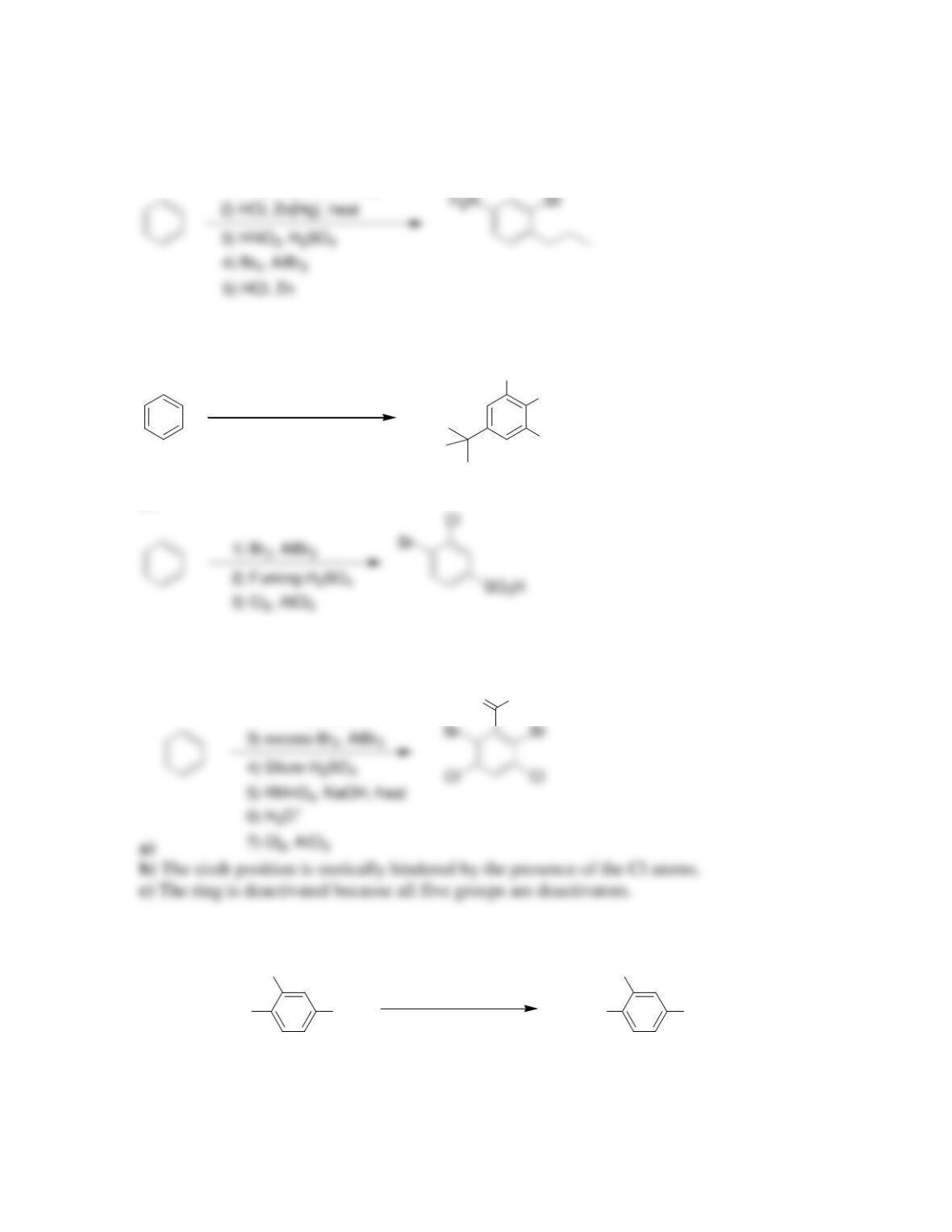
Chapter 19
Aromatic Substitution Reactions
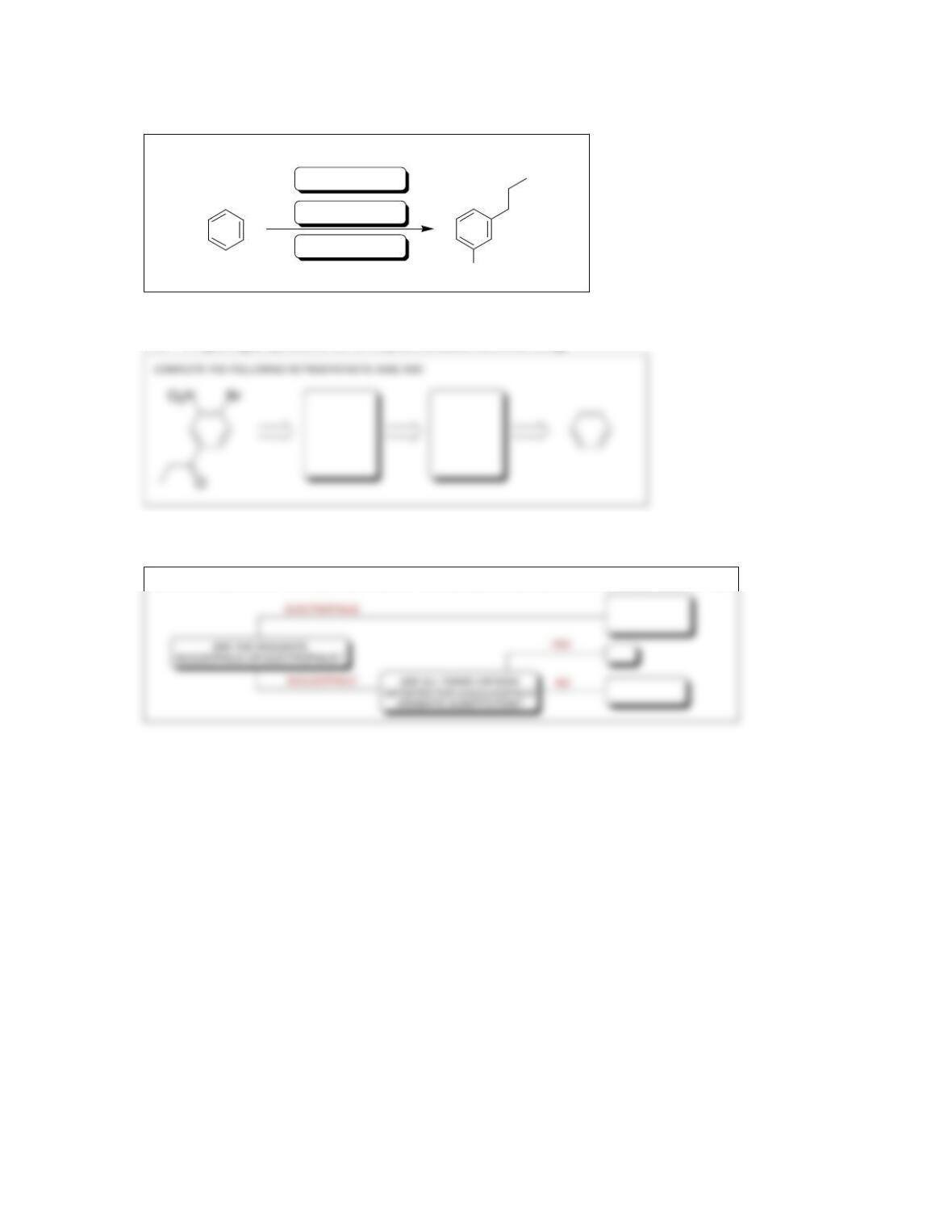
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 19. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
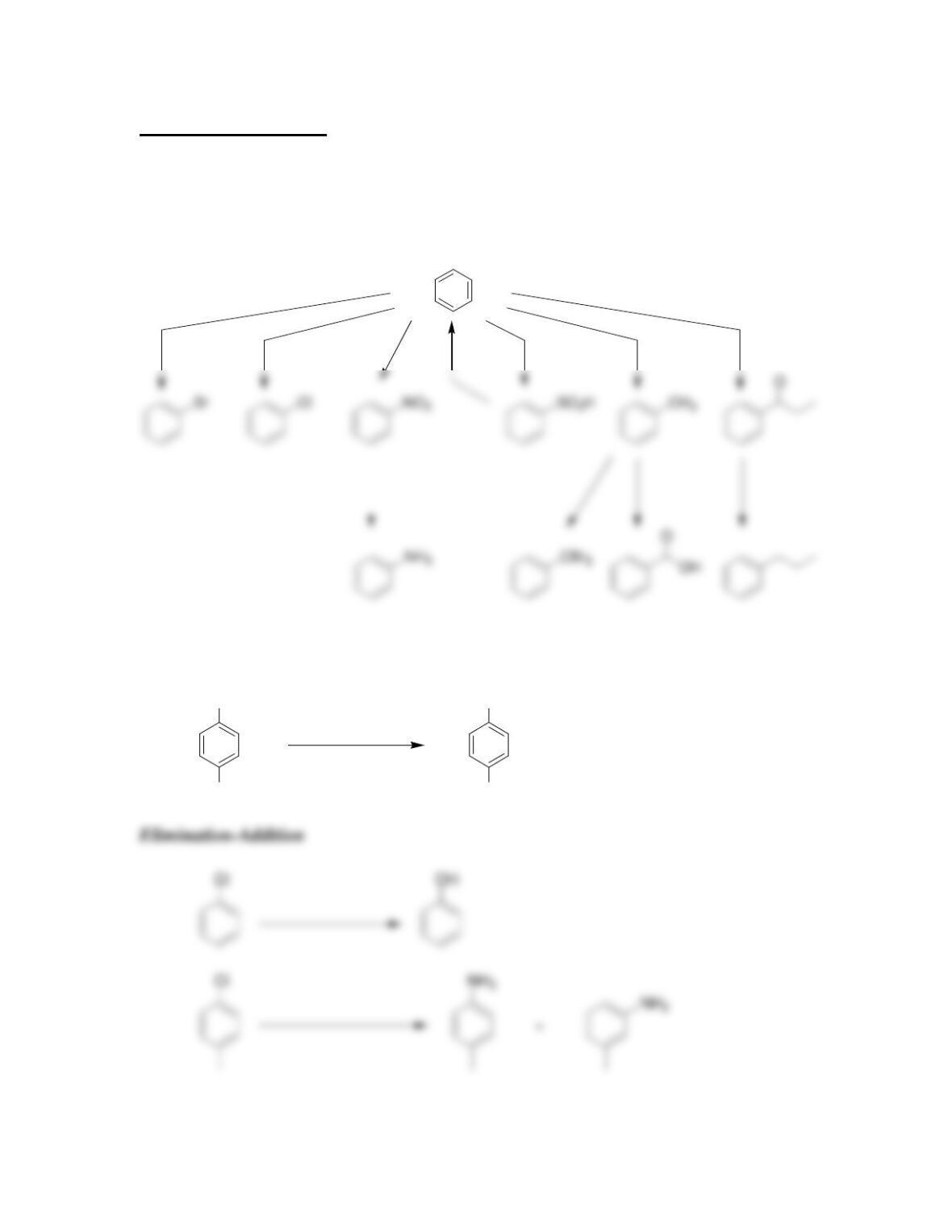
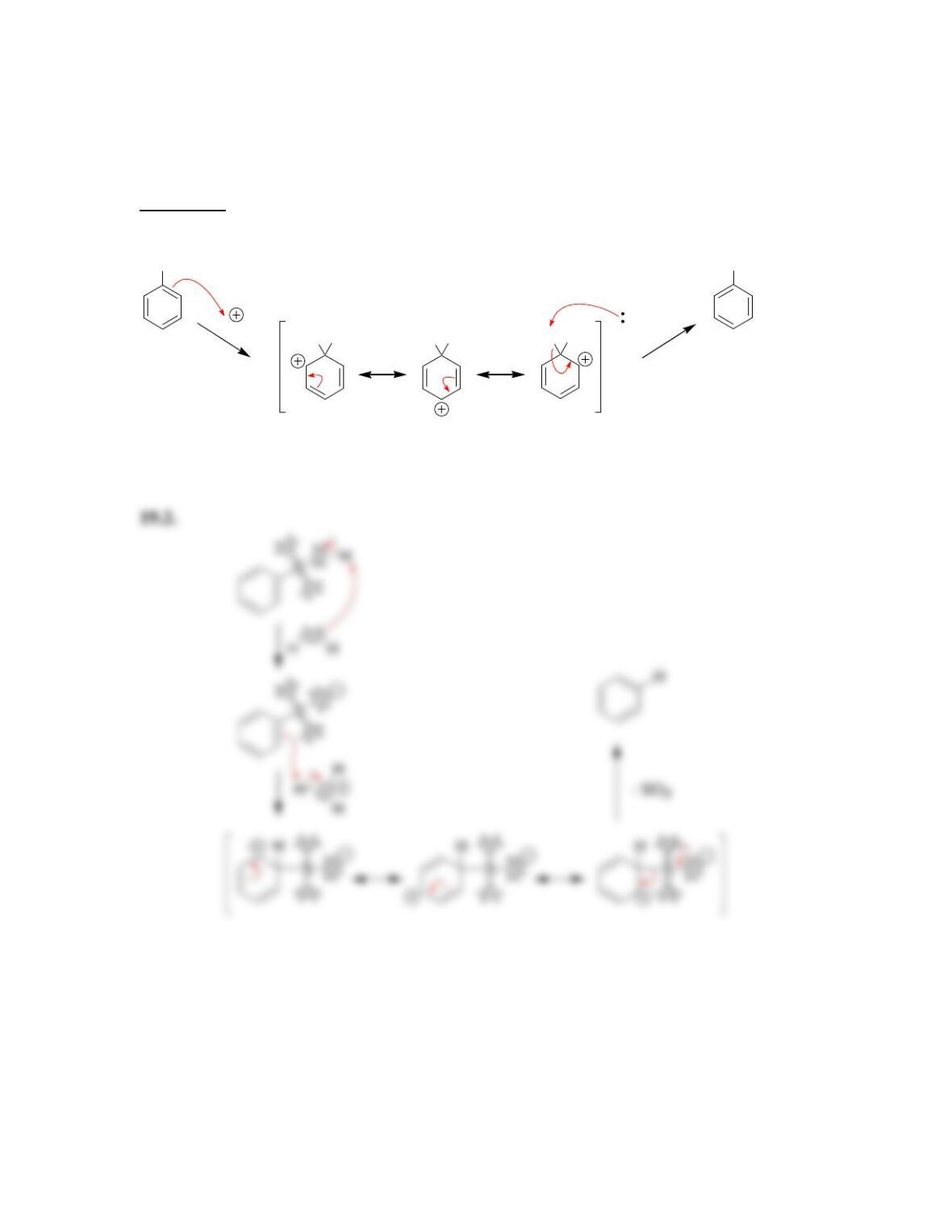
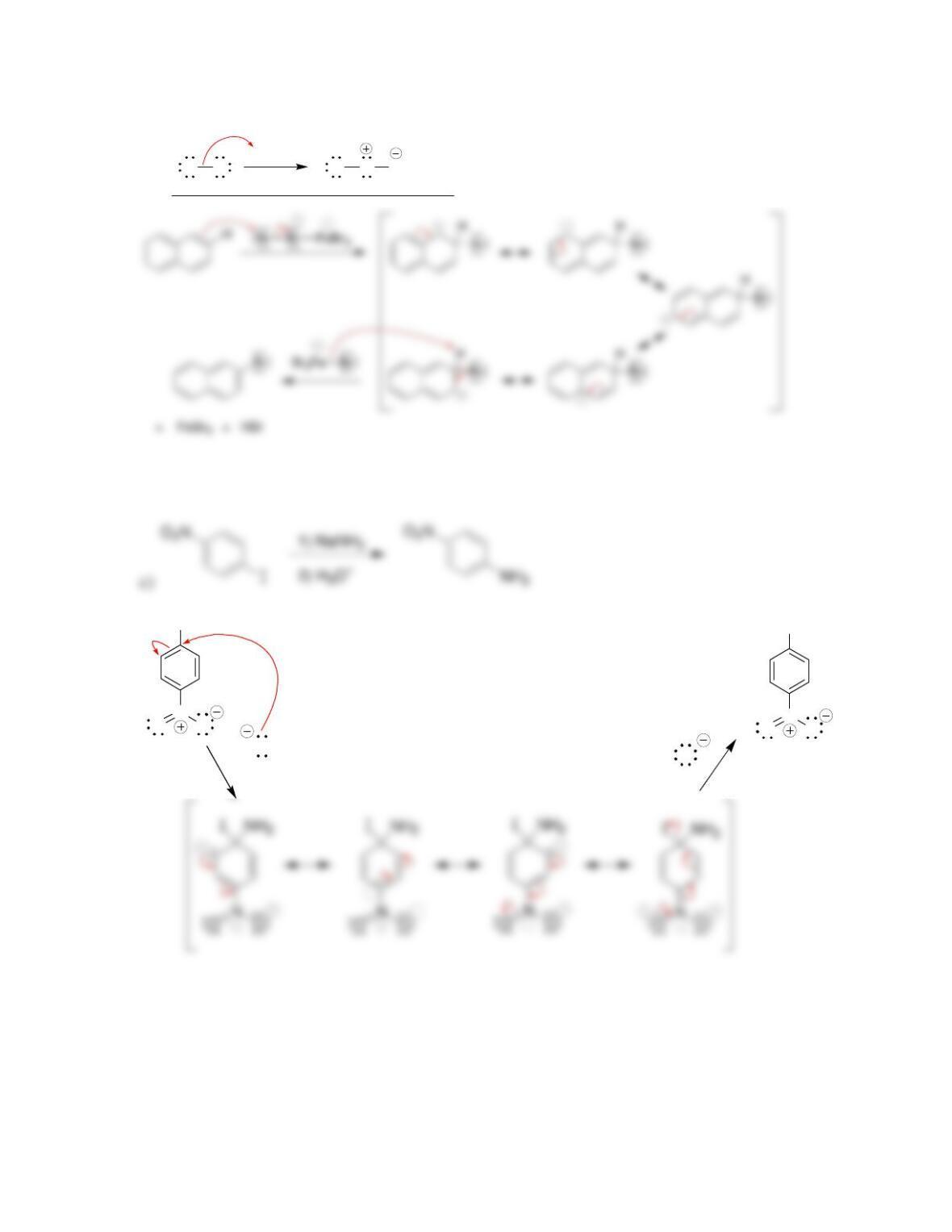
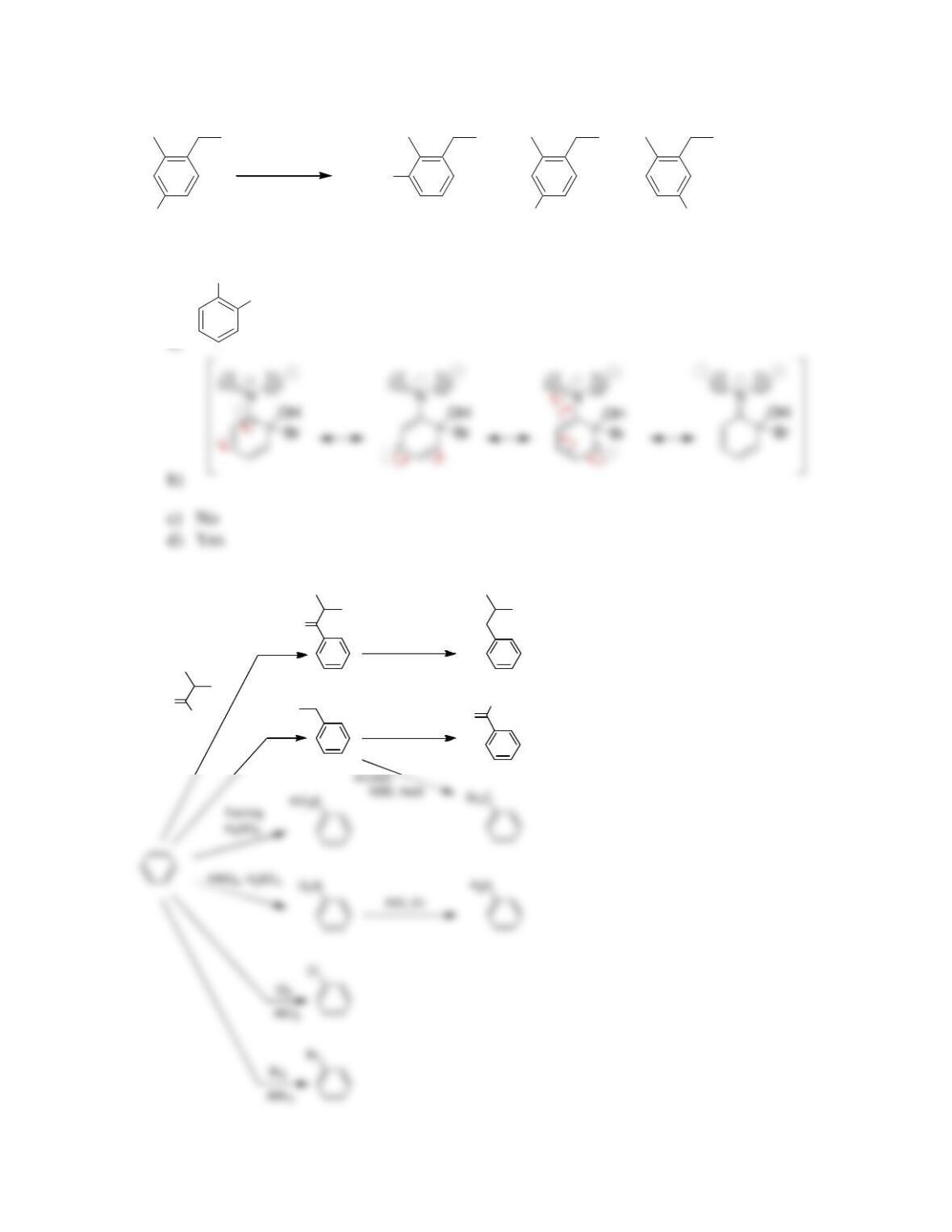
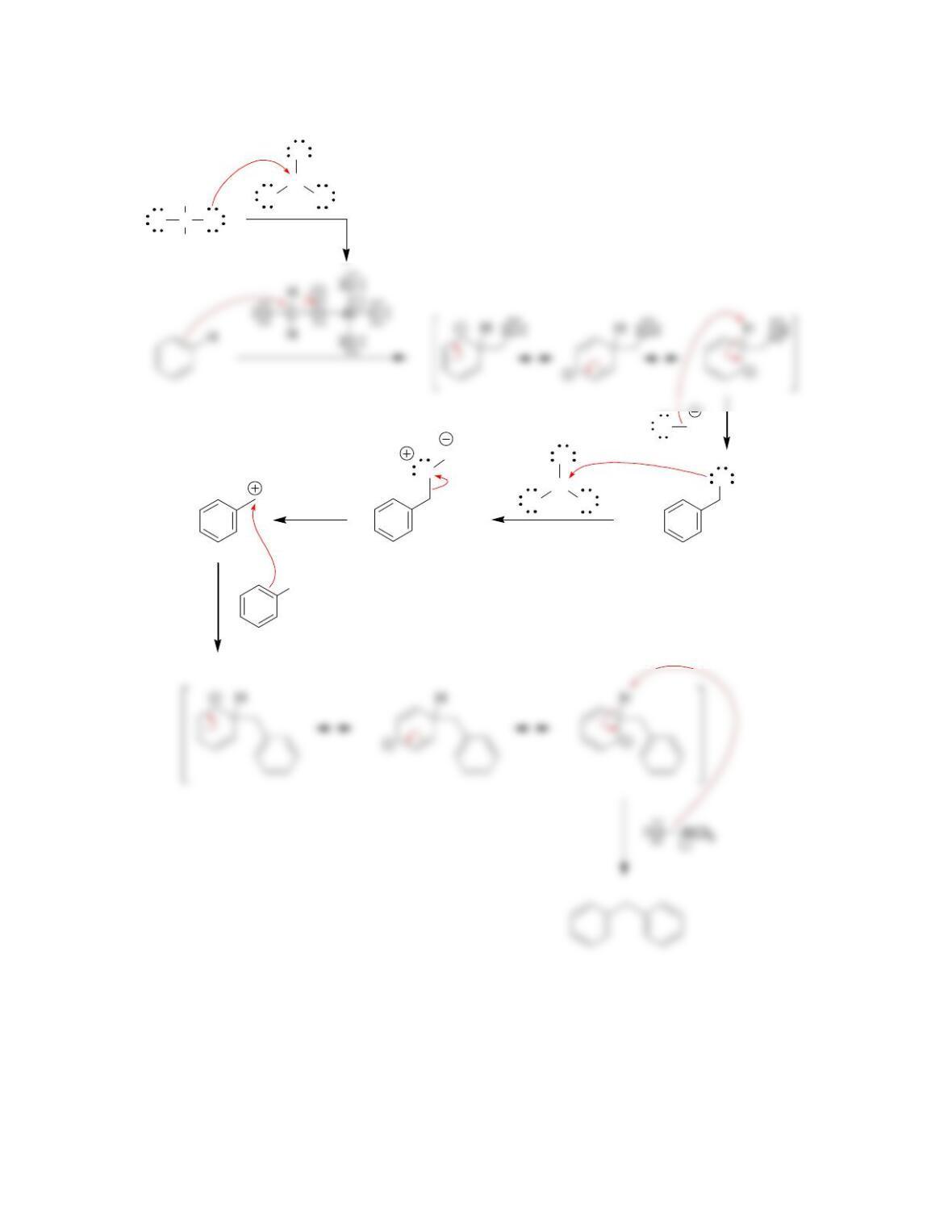
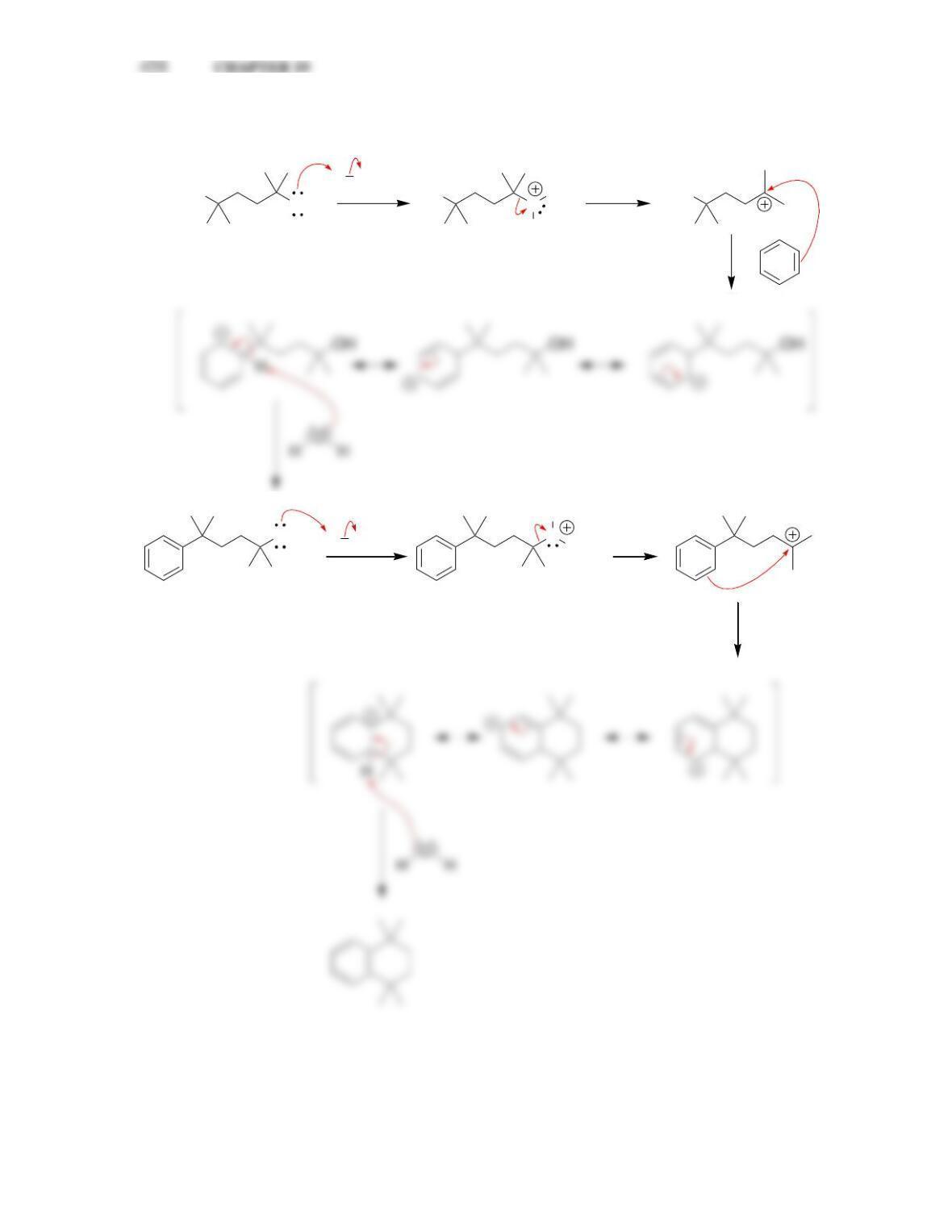
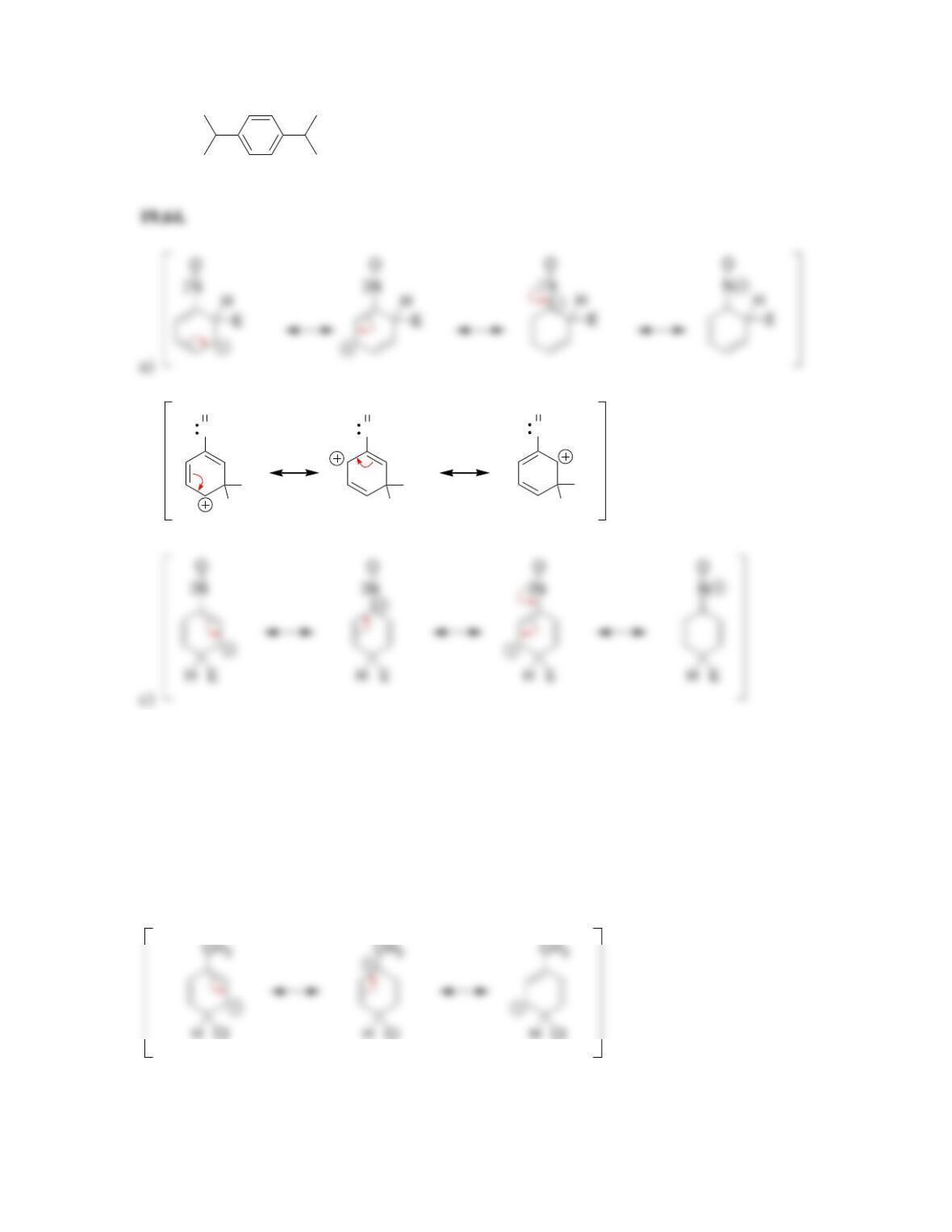
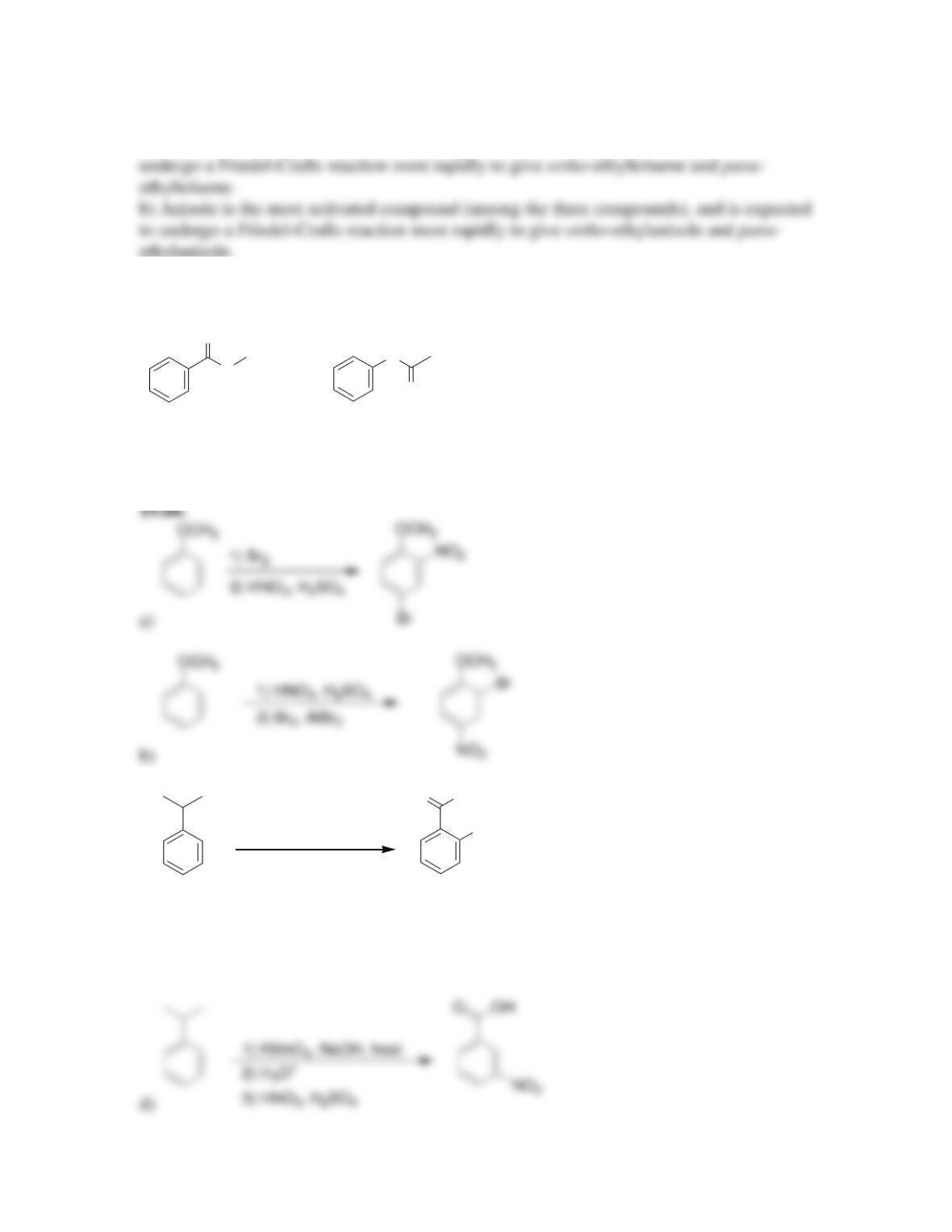
• Electrophilic aromatic substitution involves two steps:
o Formation of the __________ complex, or arenium ion.
o Deprotonation, which restores _________________.
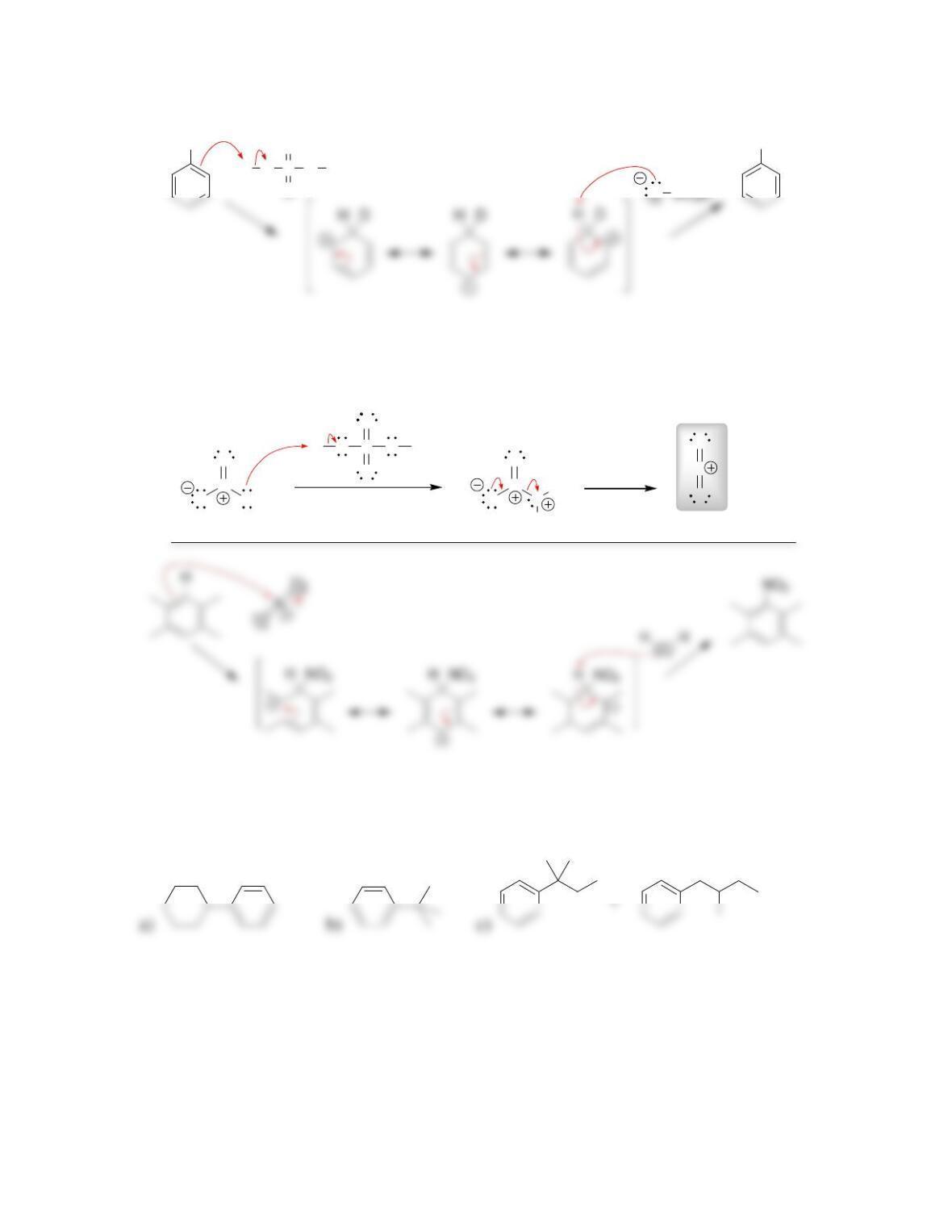
• Sulfur trioxide (SO
3
) is a very powerful ____________ that is present in fuming
sulfuric acid. Benzene reacts with SO
3
in a reversible process called __________.
• A mixture of sulfuric acid and nitric acid produces the nitronium ion (NO
2+
).
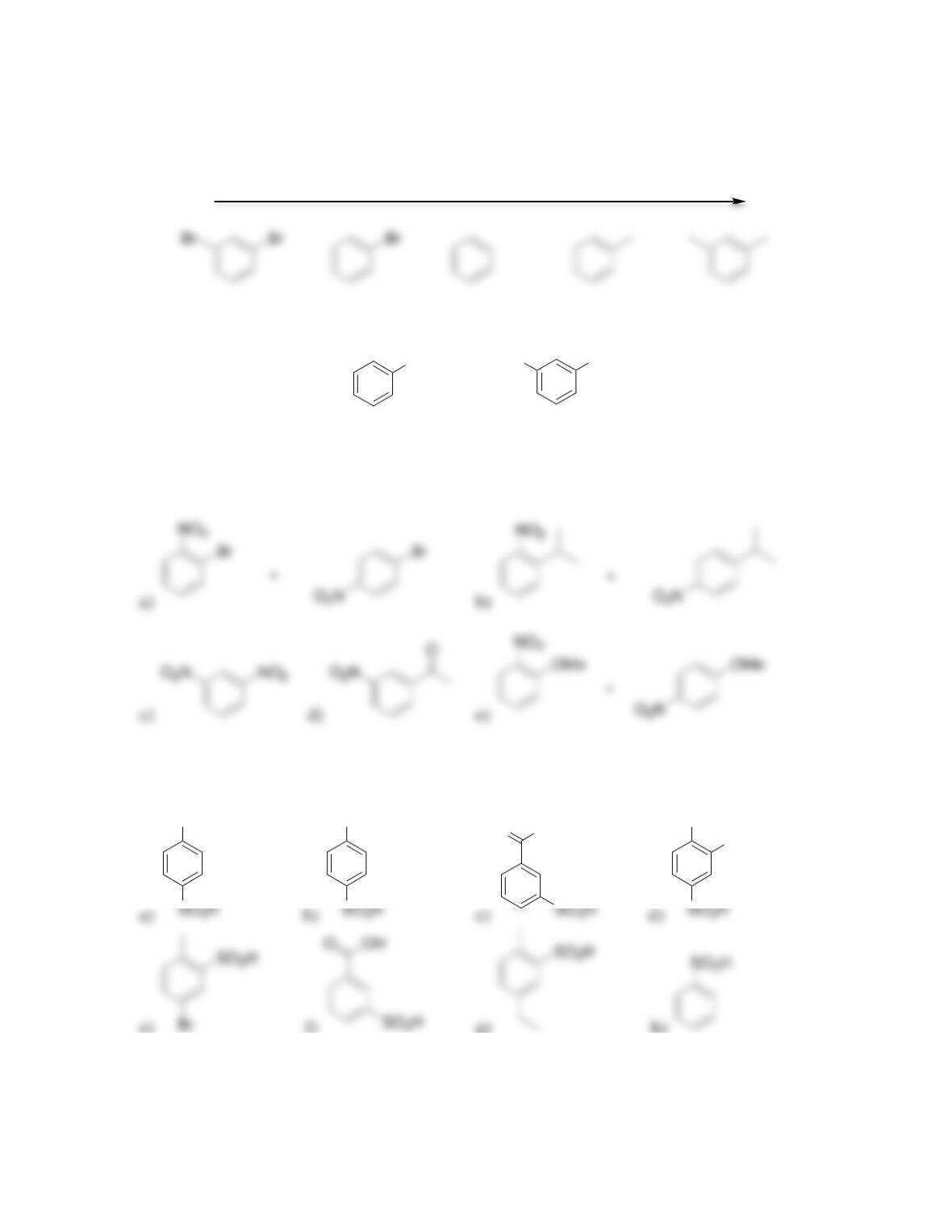
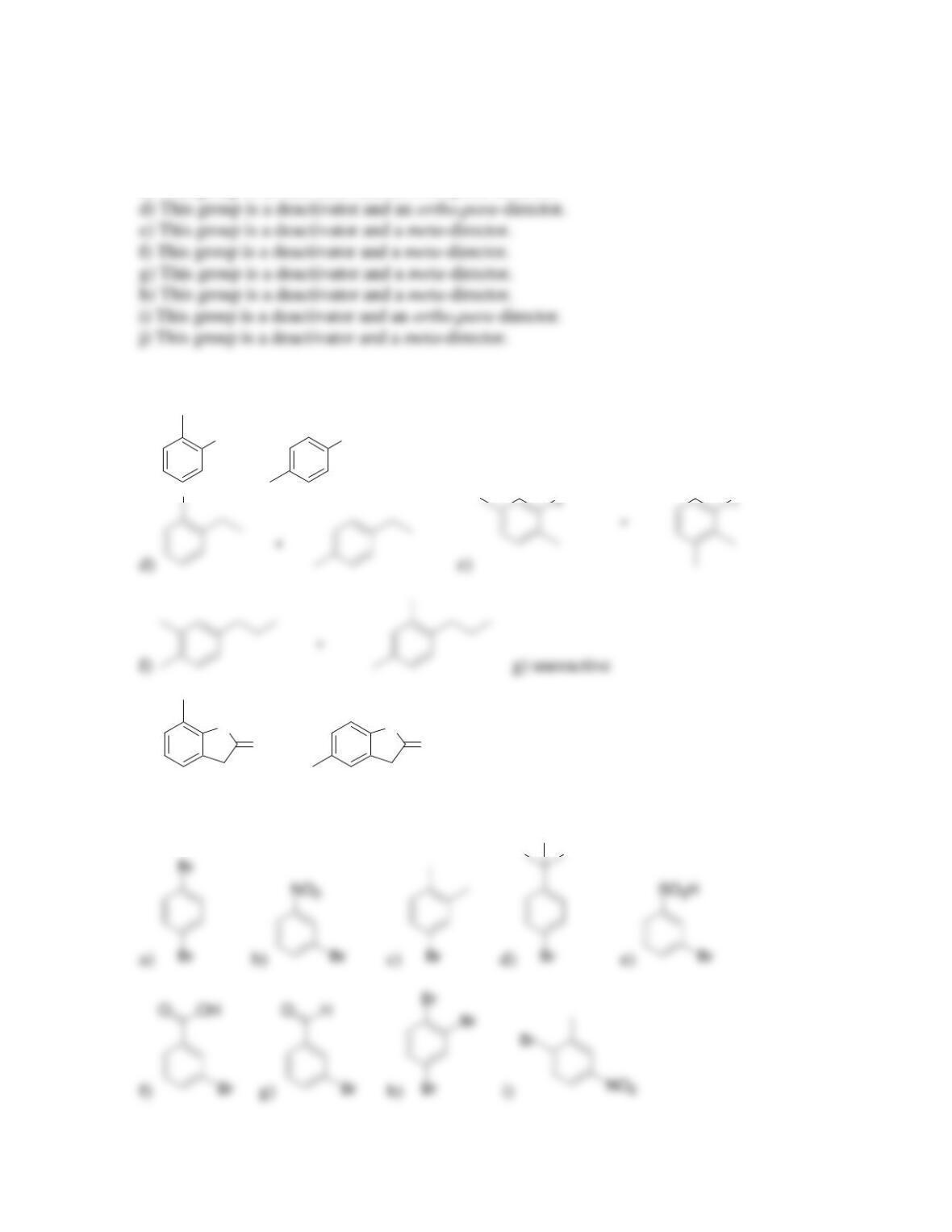
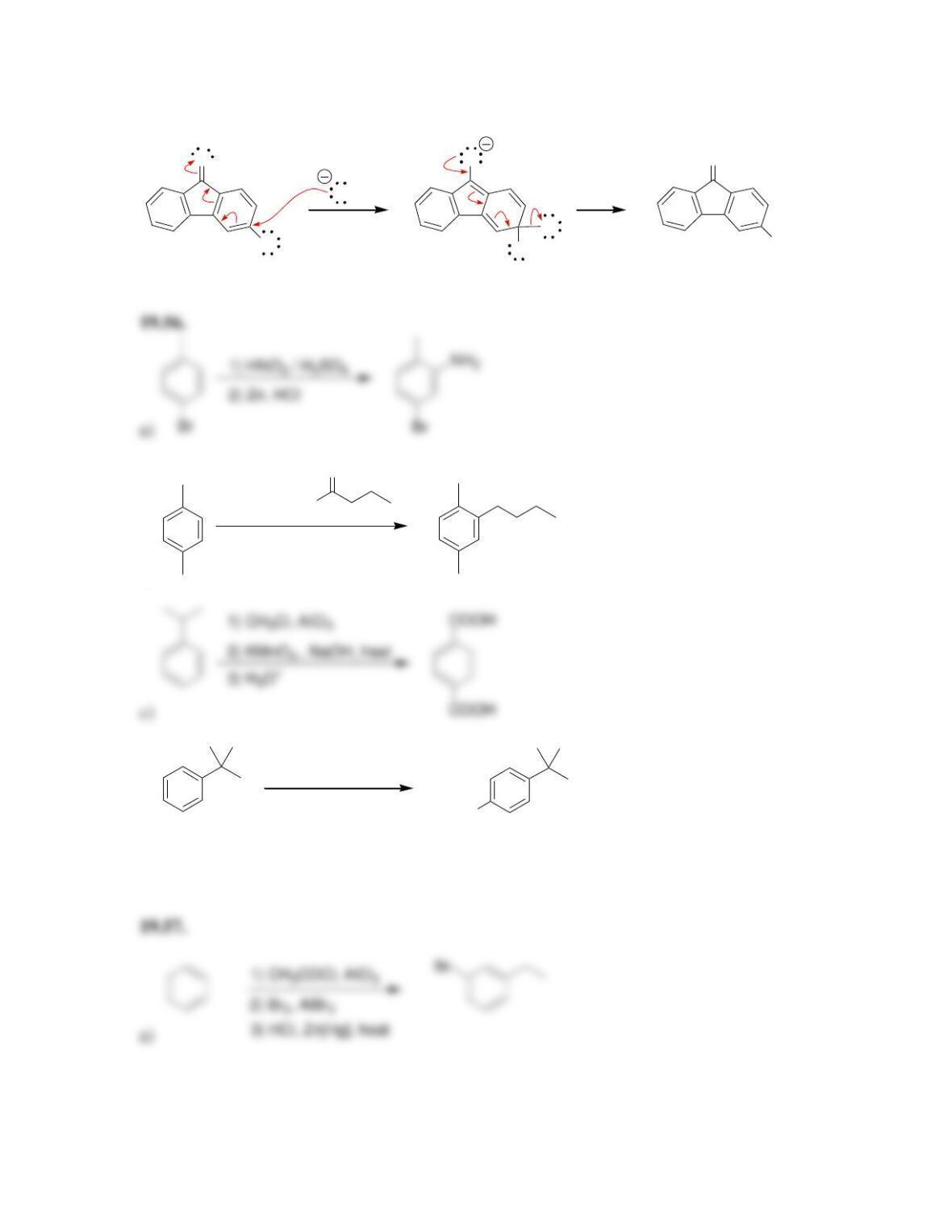
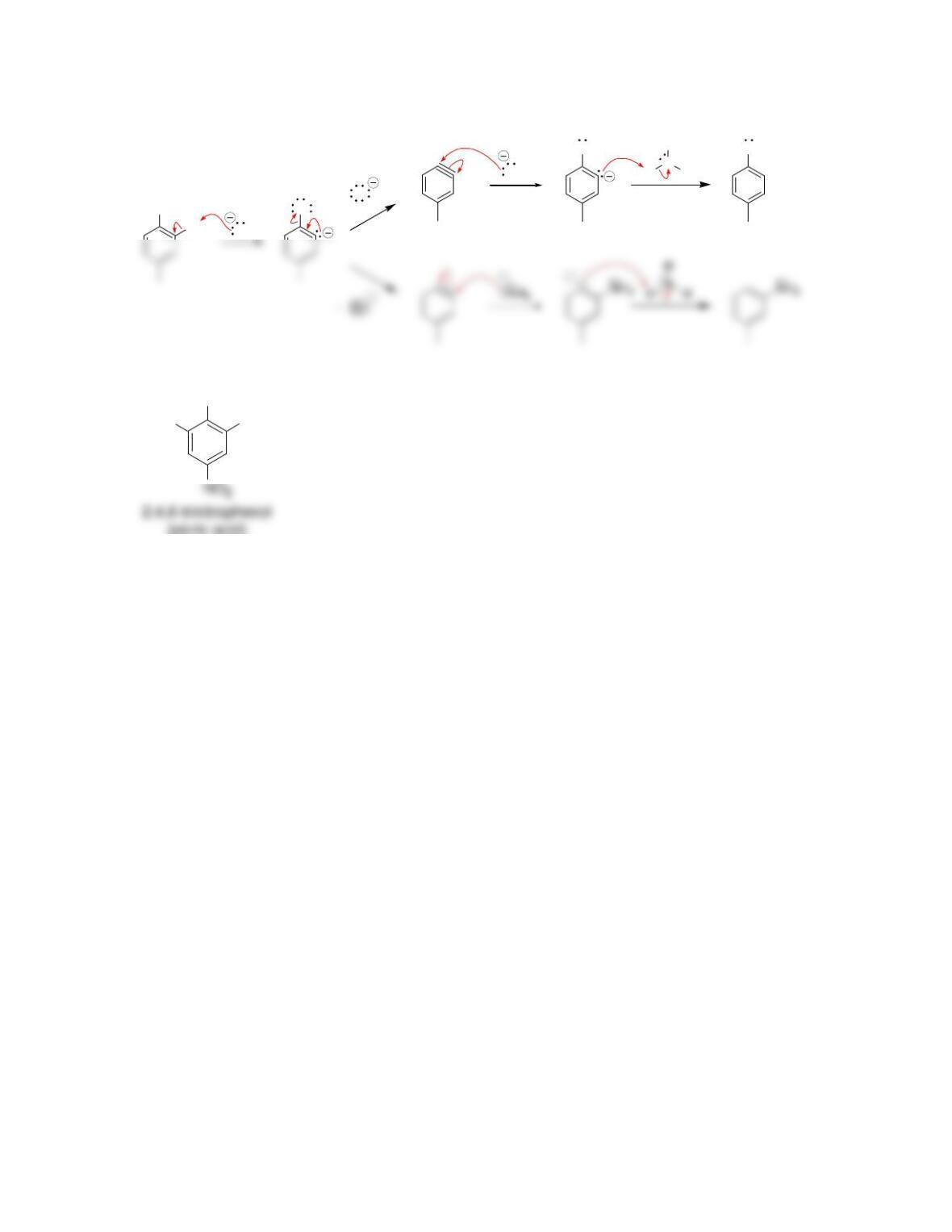
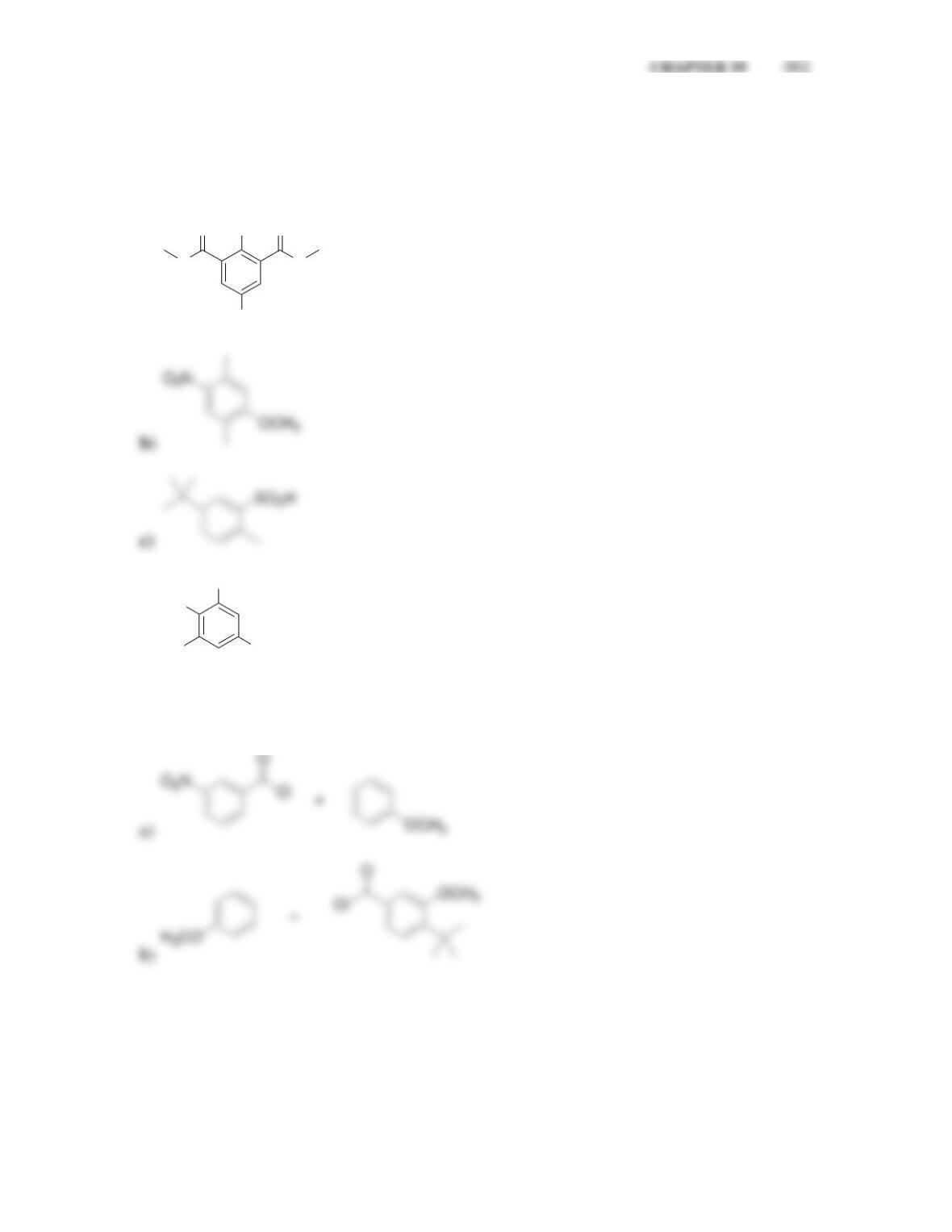
director.
• All activators are ______-______directors
• A nitro group deactivates an aromatic ring and is a ______director.
• Most deactivators are ______directors.
• Strong activators are characterized by the presence of a __________________
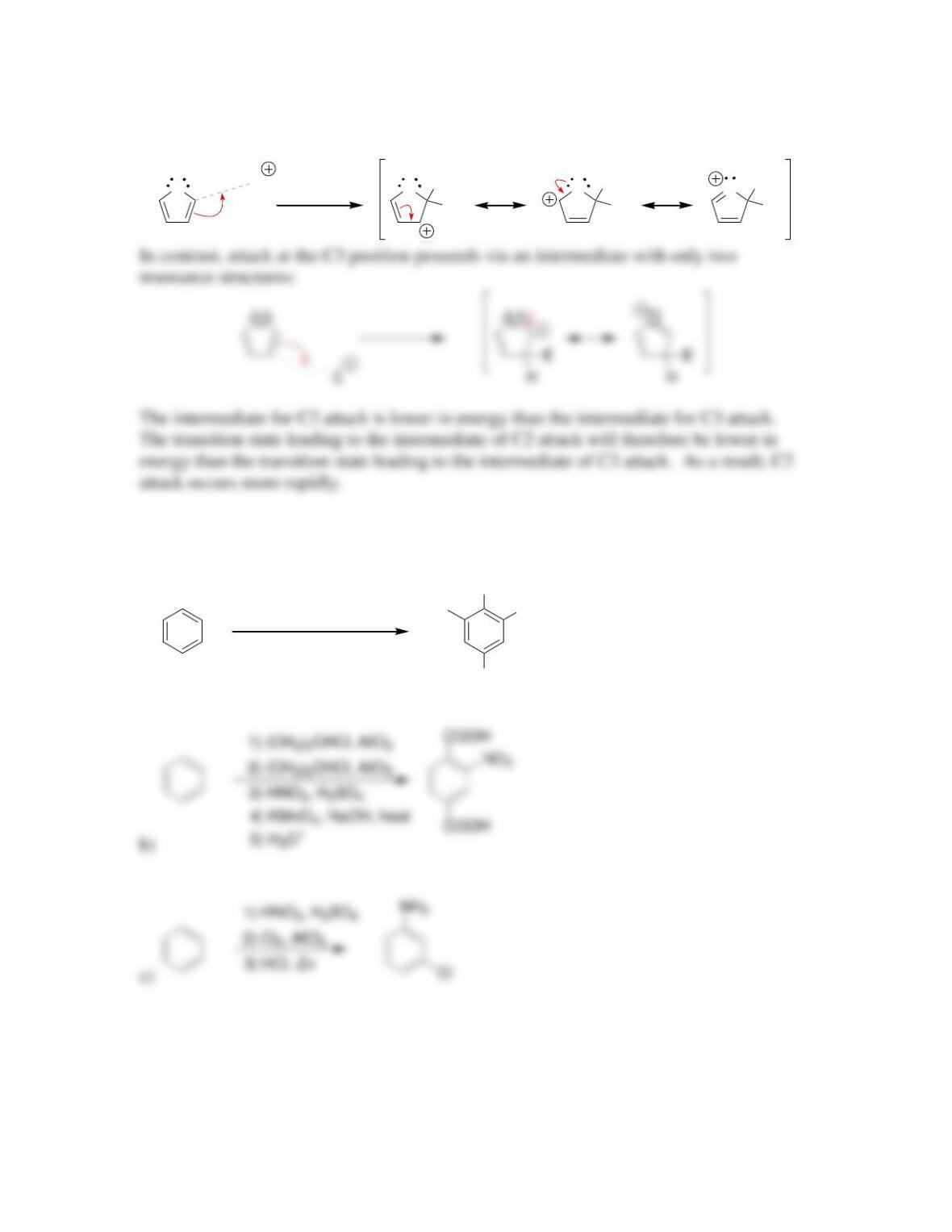
• An elimination-addition reaction occurs via a ____________intermediate.