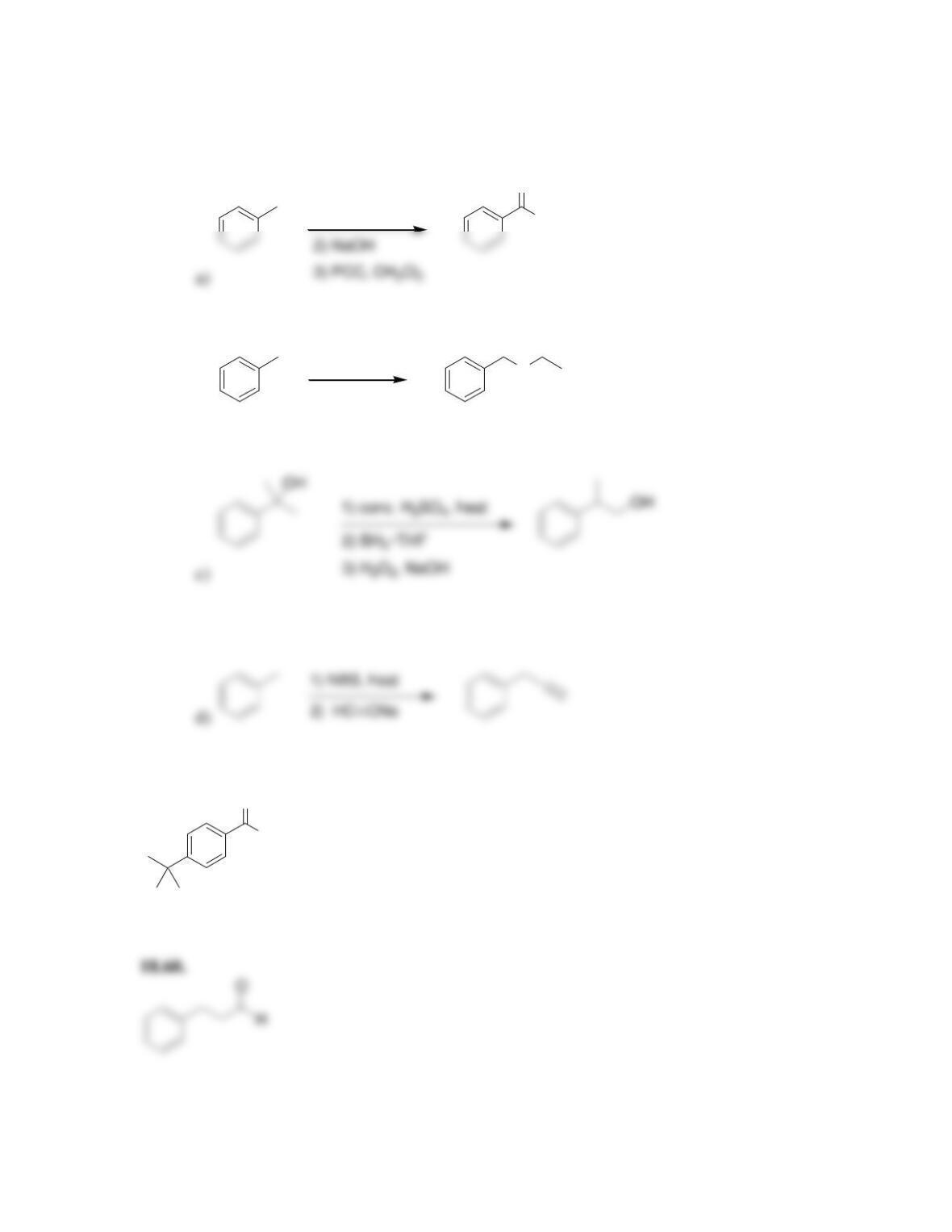
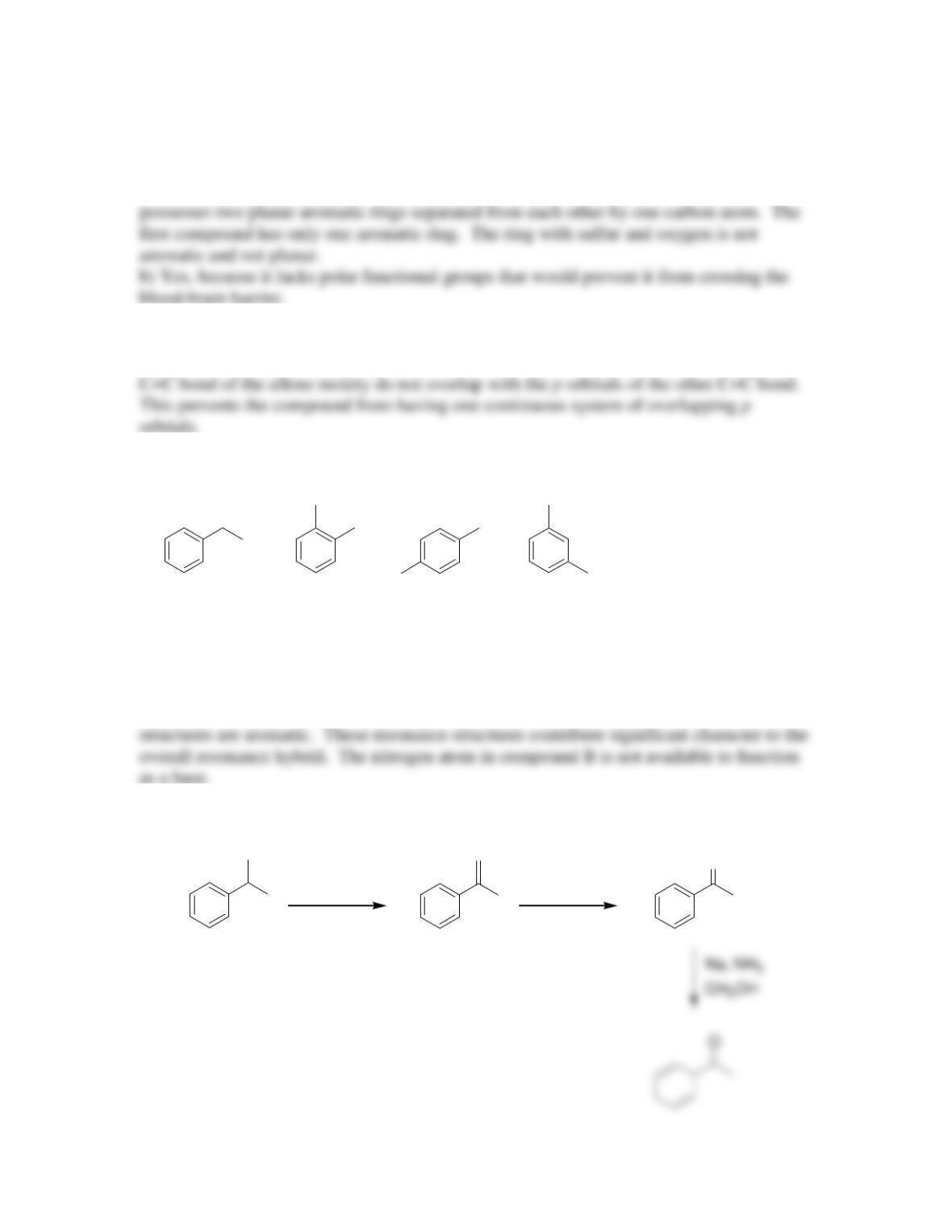
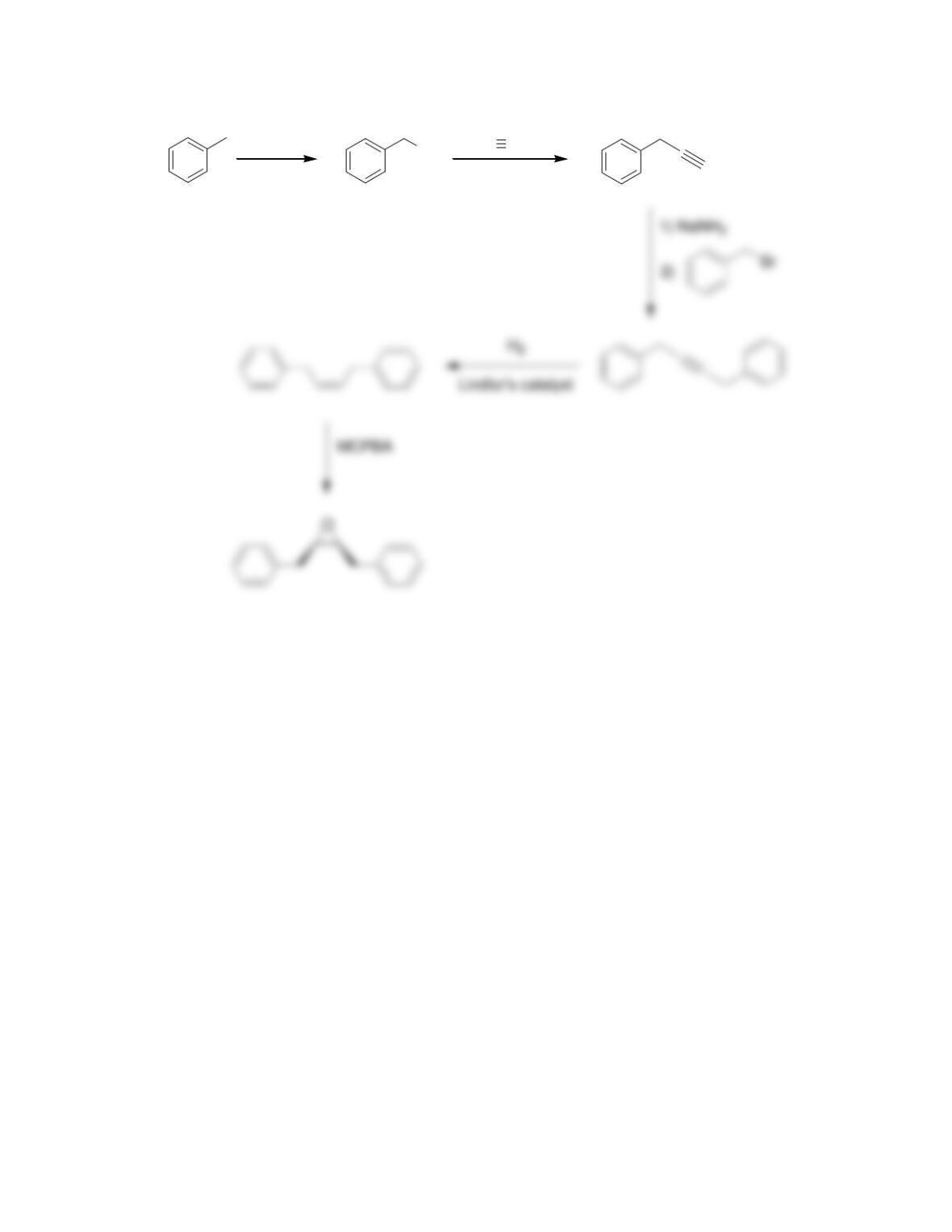
CHAPTER 18
415
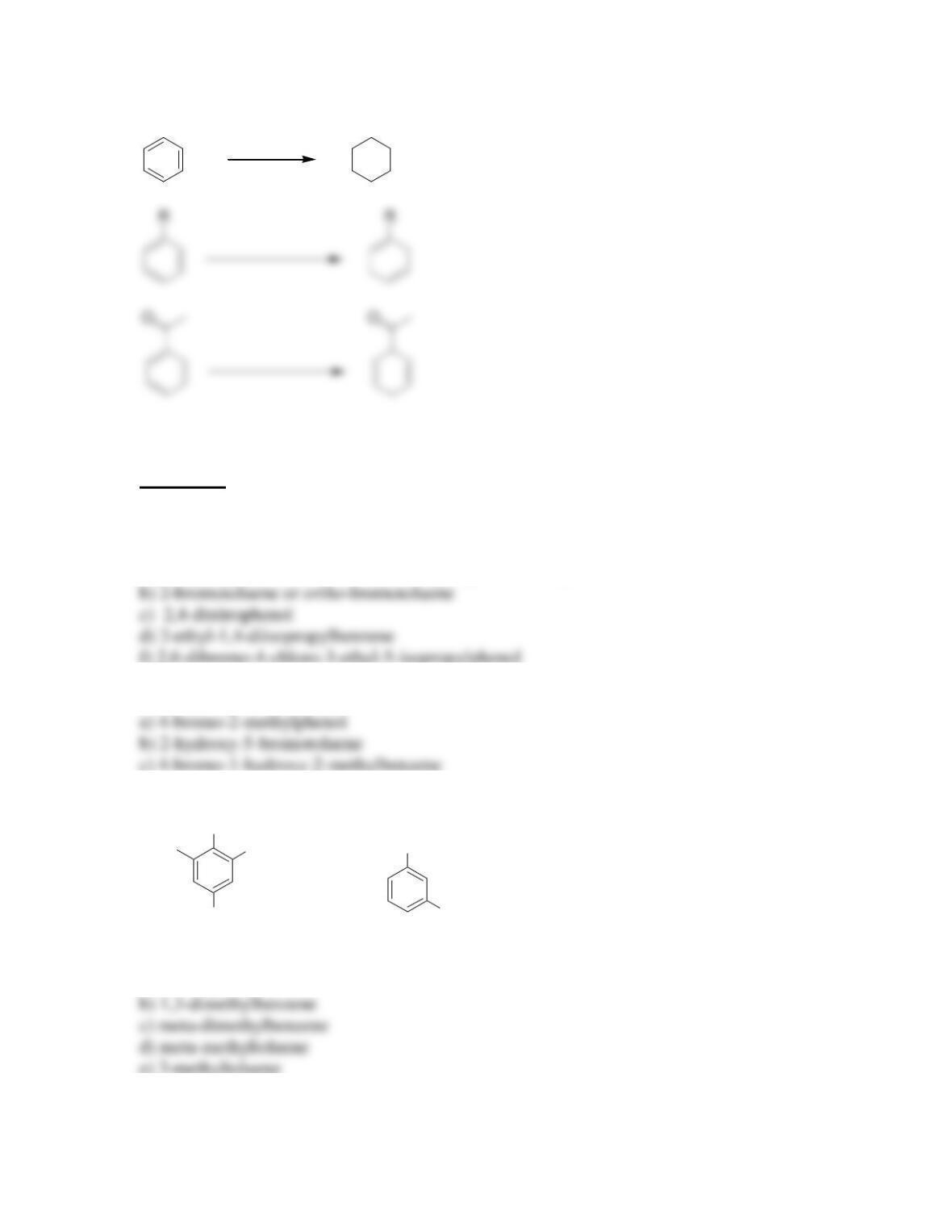
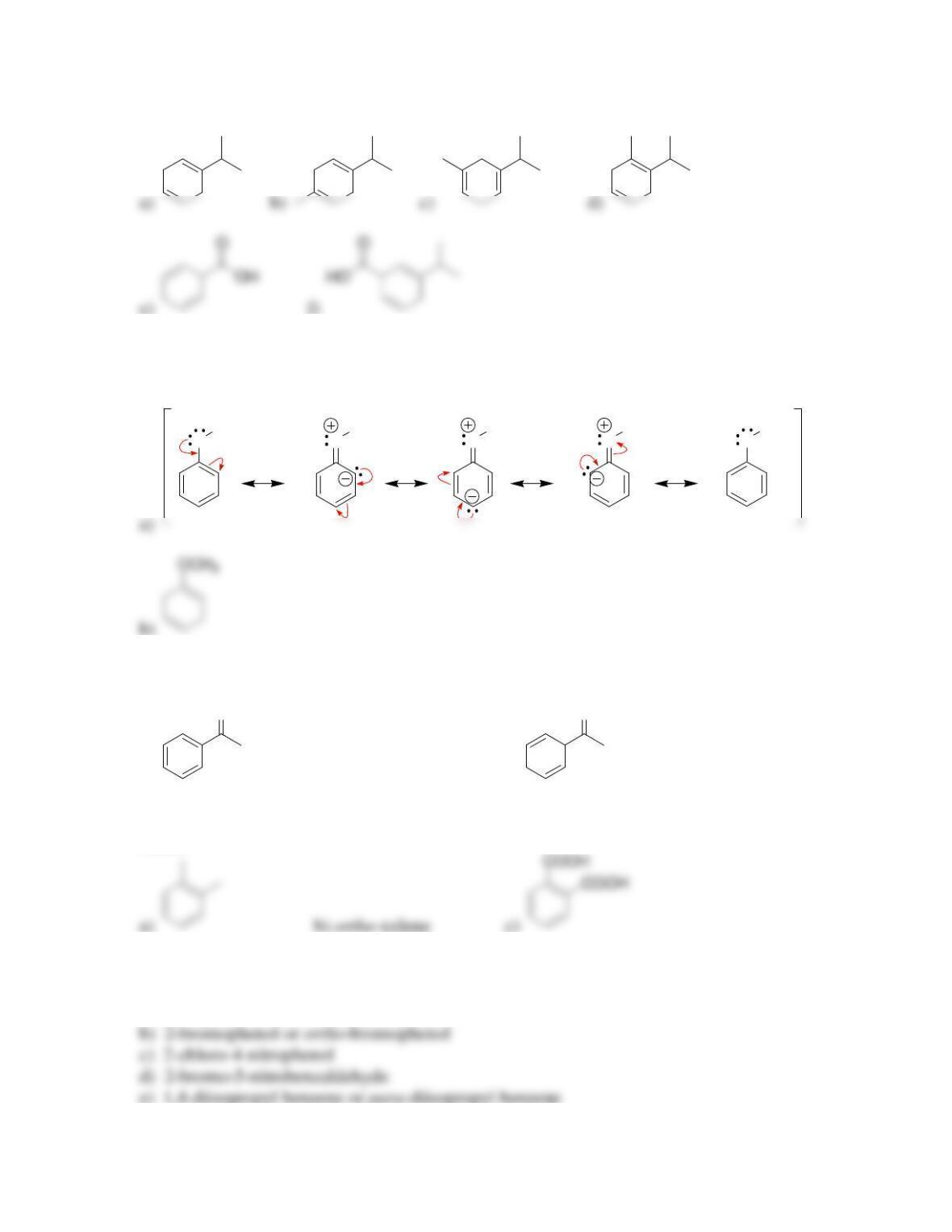
18.12. Cyclopentadiene is more acidic because its conjugate base is highly stabilized.
Deprotonation of cyclopentadiene generates an anion that is aromatic, because it is a
continuous system of overlapping p orbitals containing 6 π electrons. In contrast,
deprotonation of cycloheptatriene gives an anion with 8 π electrons.
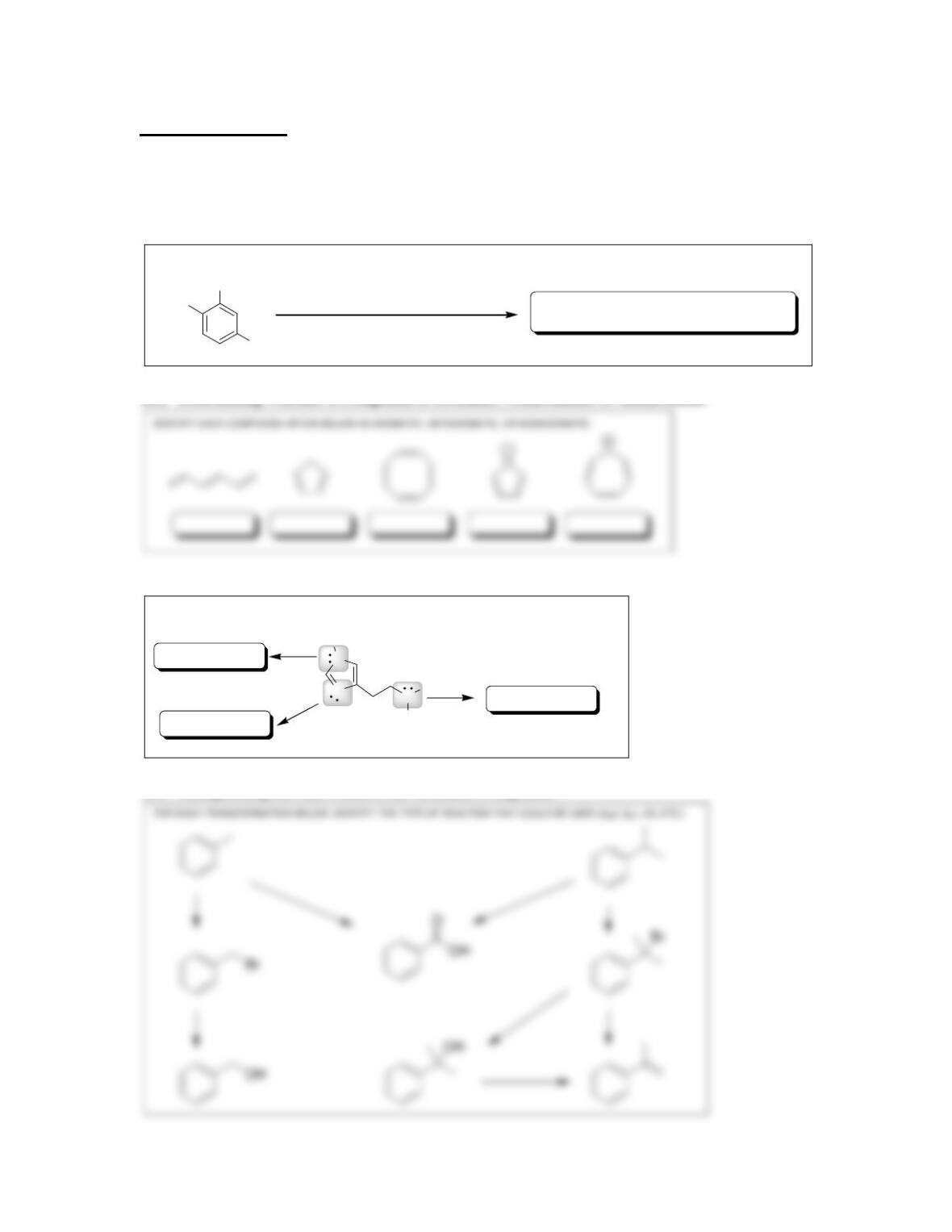
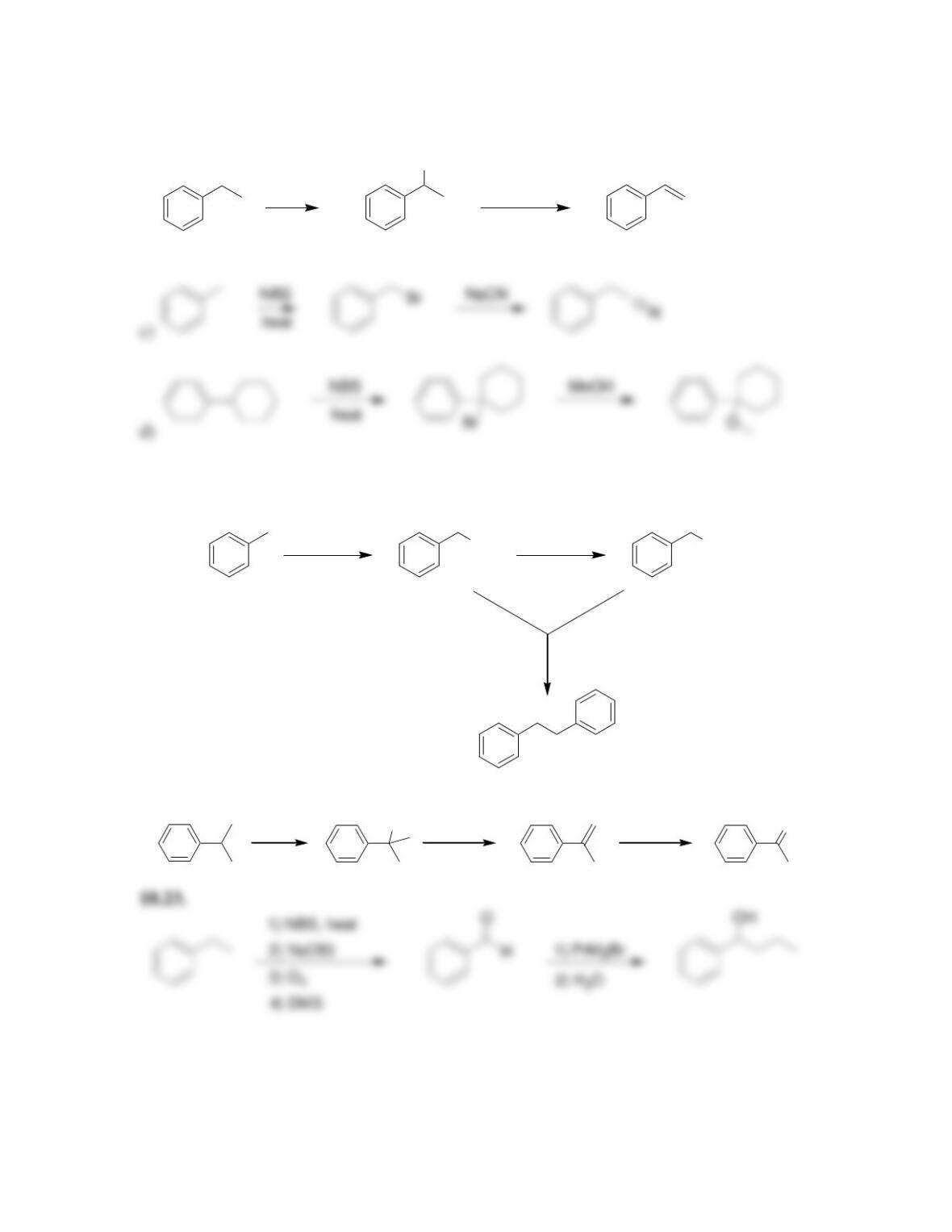
18.13. The first step of an S
N
1 process is loss of a leaving group, forming a carbocation,
so we compare the carbocations that would be formed.
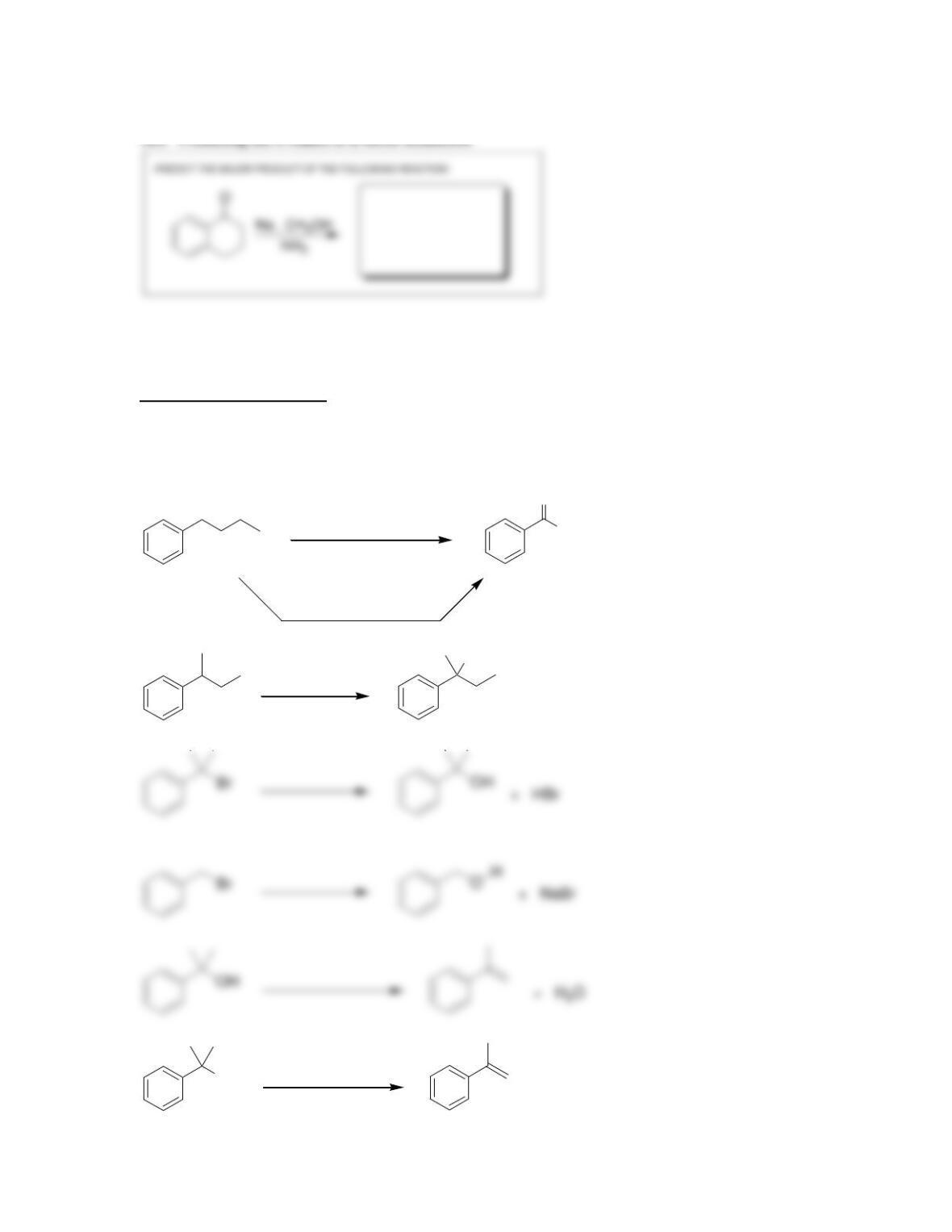
18.14. The first compound is more acidic because deprotonation of the first compound
generates a new (second) aromatic ring. Deprotonation of the second compound does not
introduce a new aromatic ring:
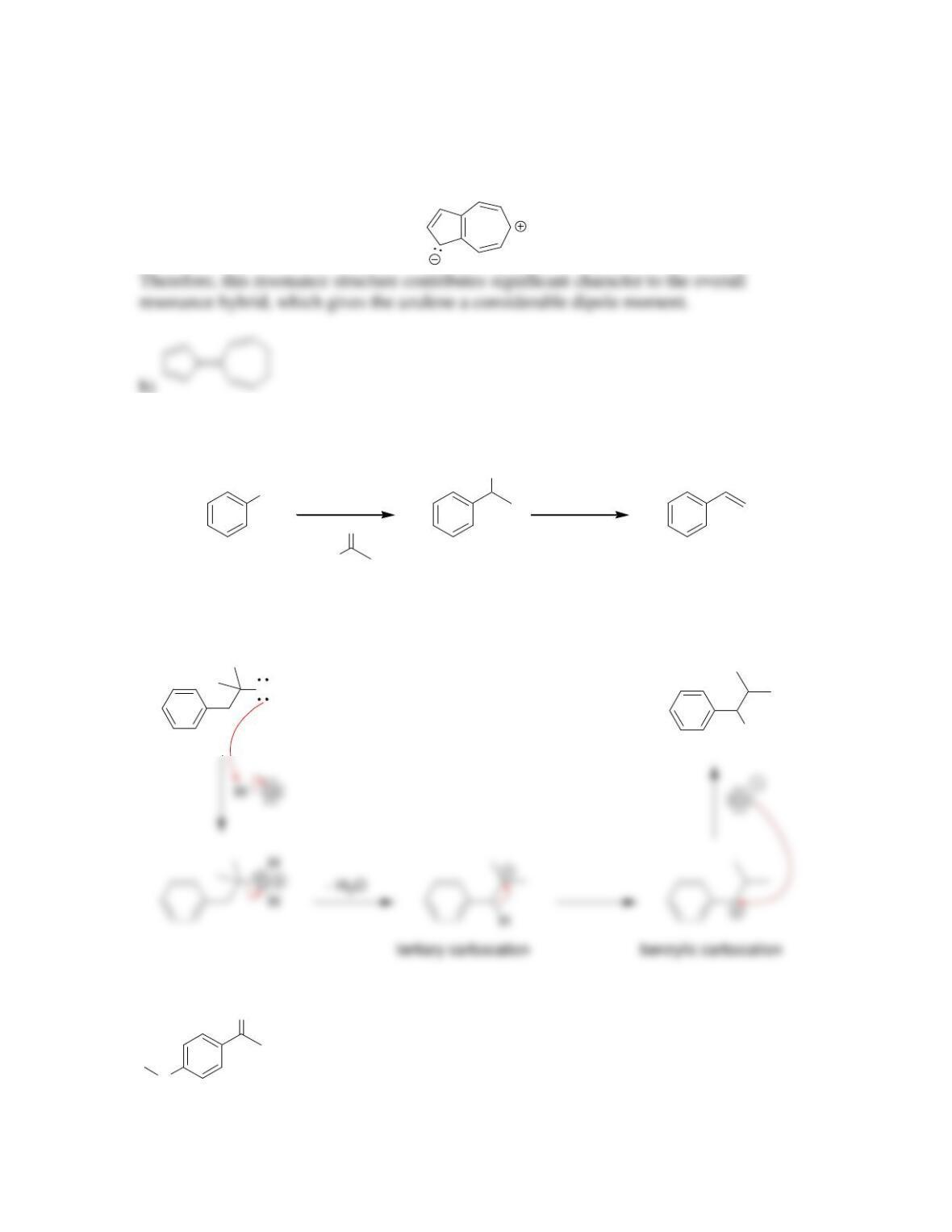
18.15.
a) One of the lone pairs on oxygen
b) One of the lone pairs on sulfur