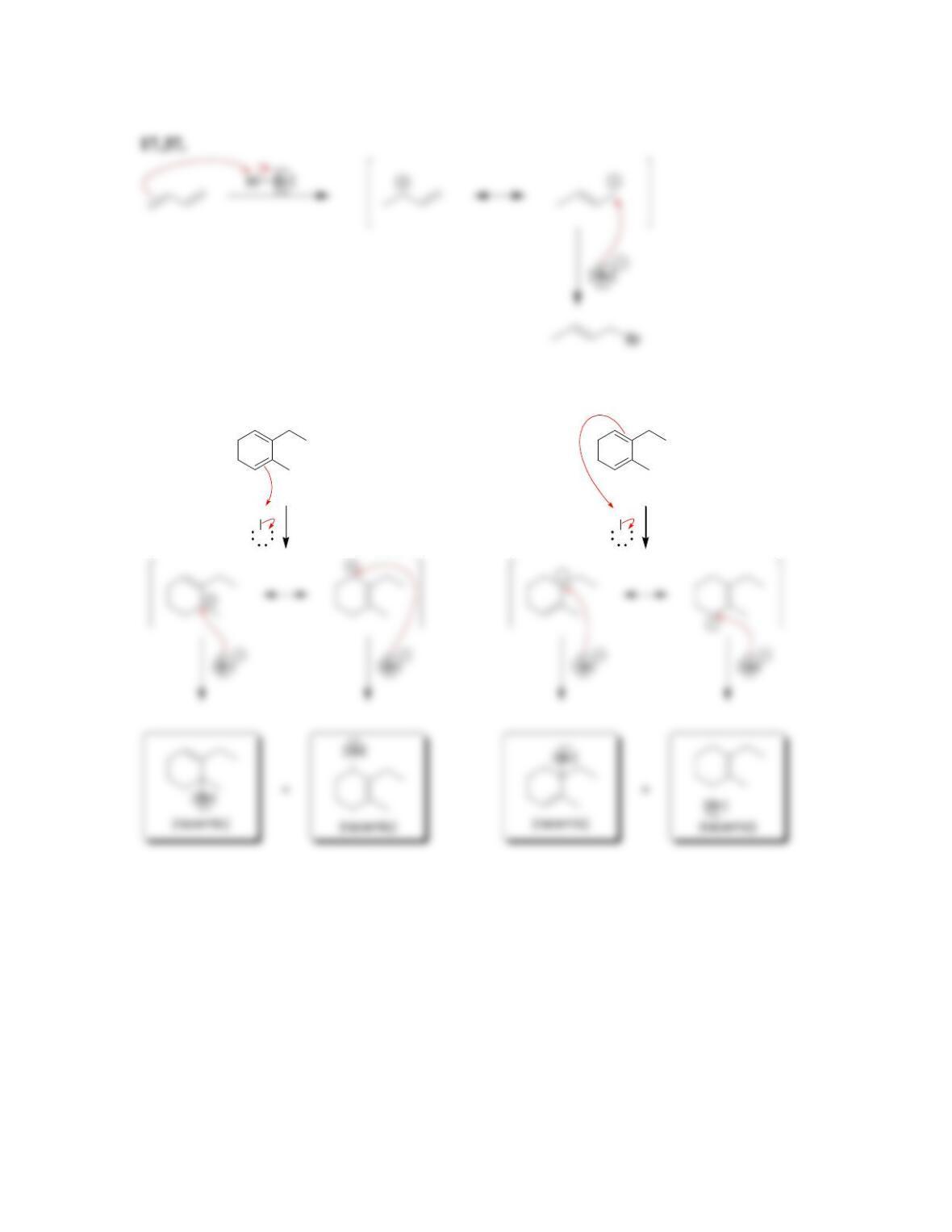
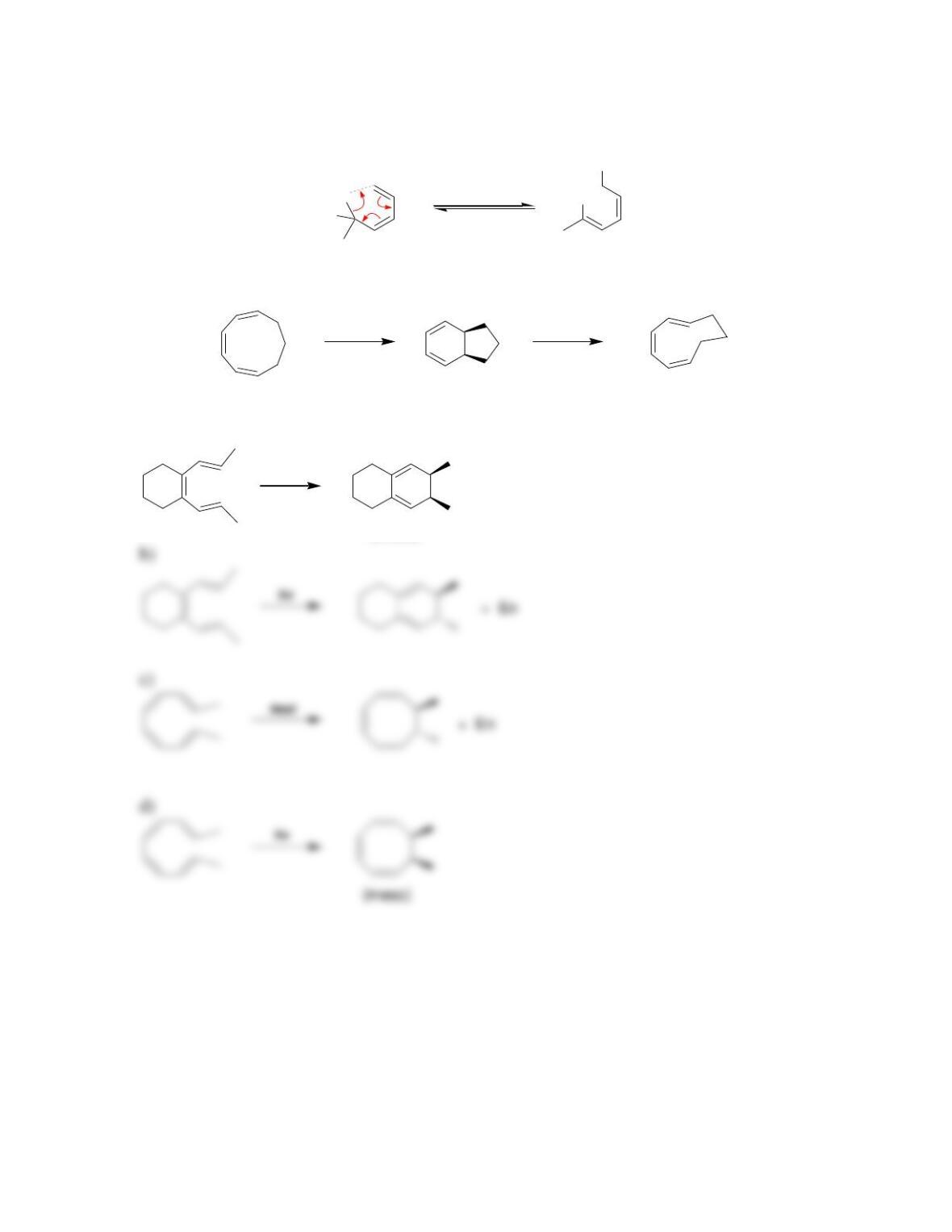
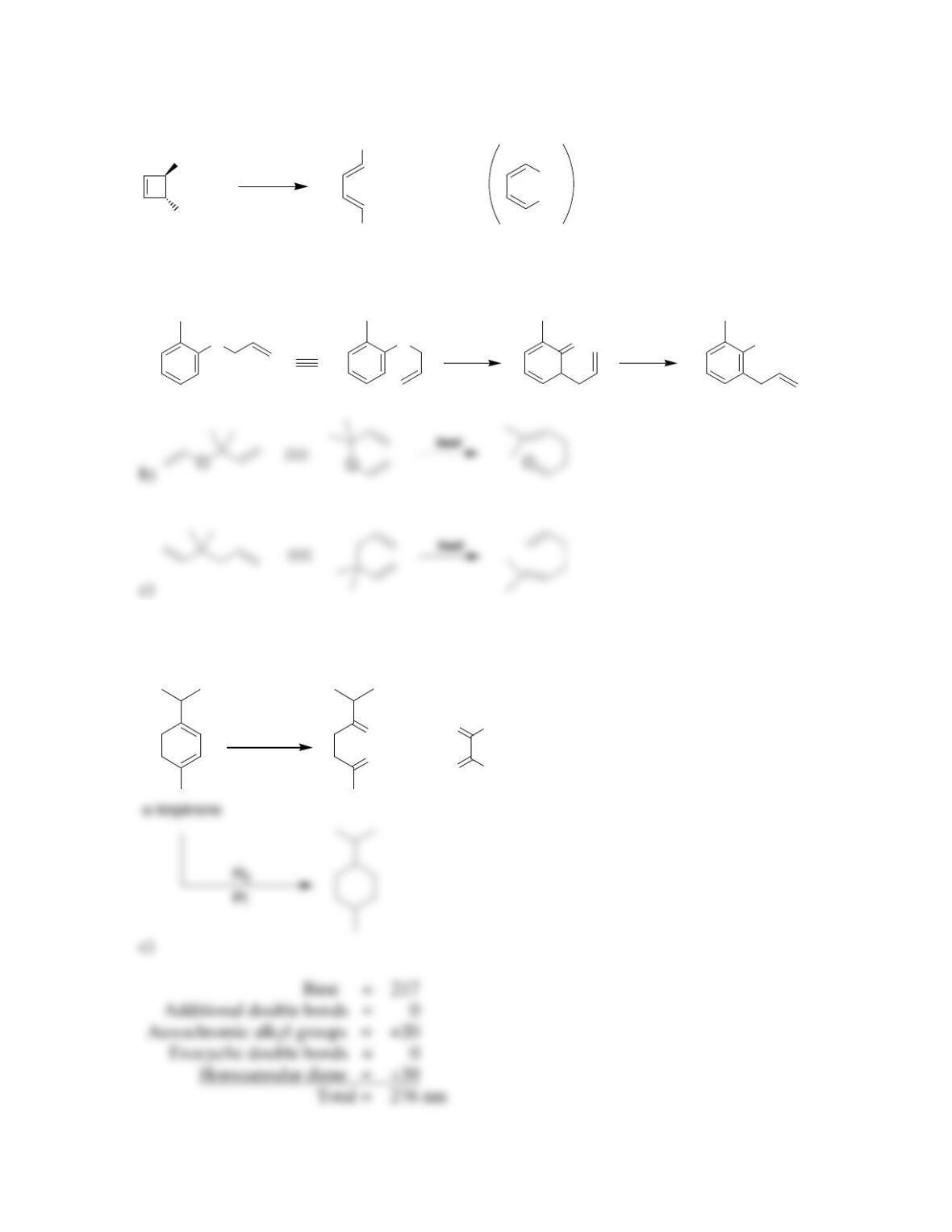
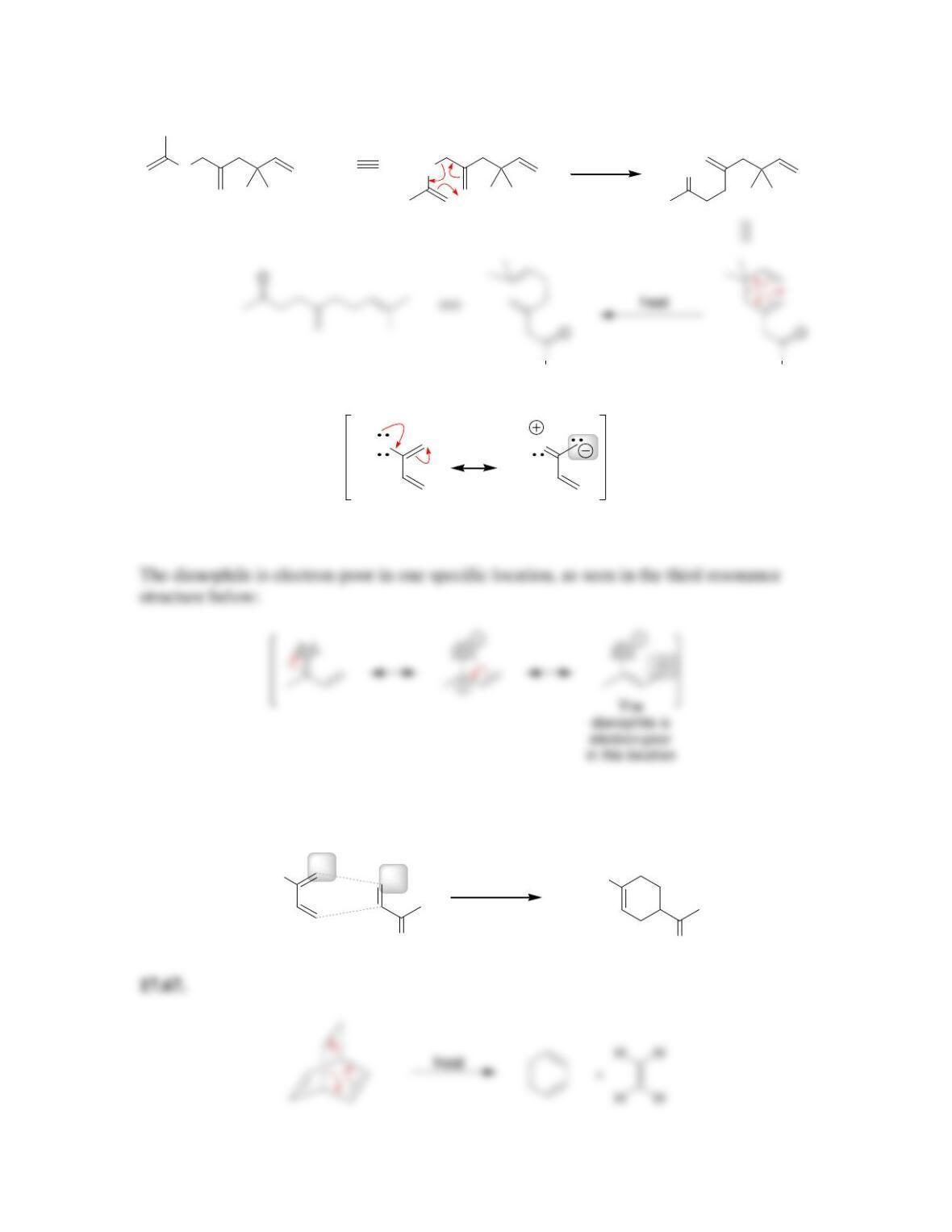
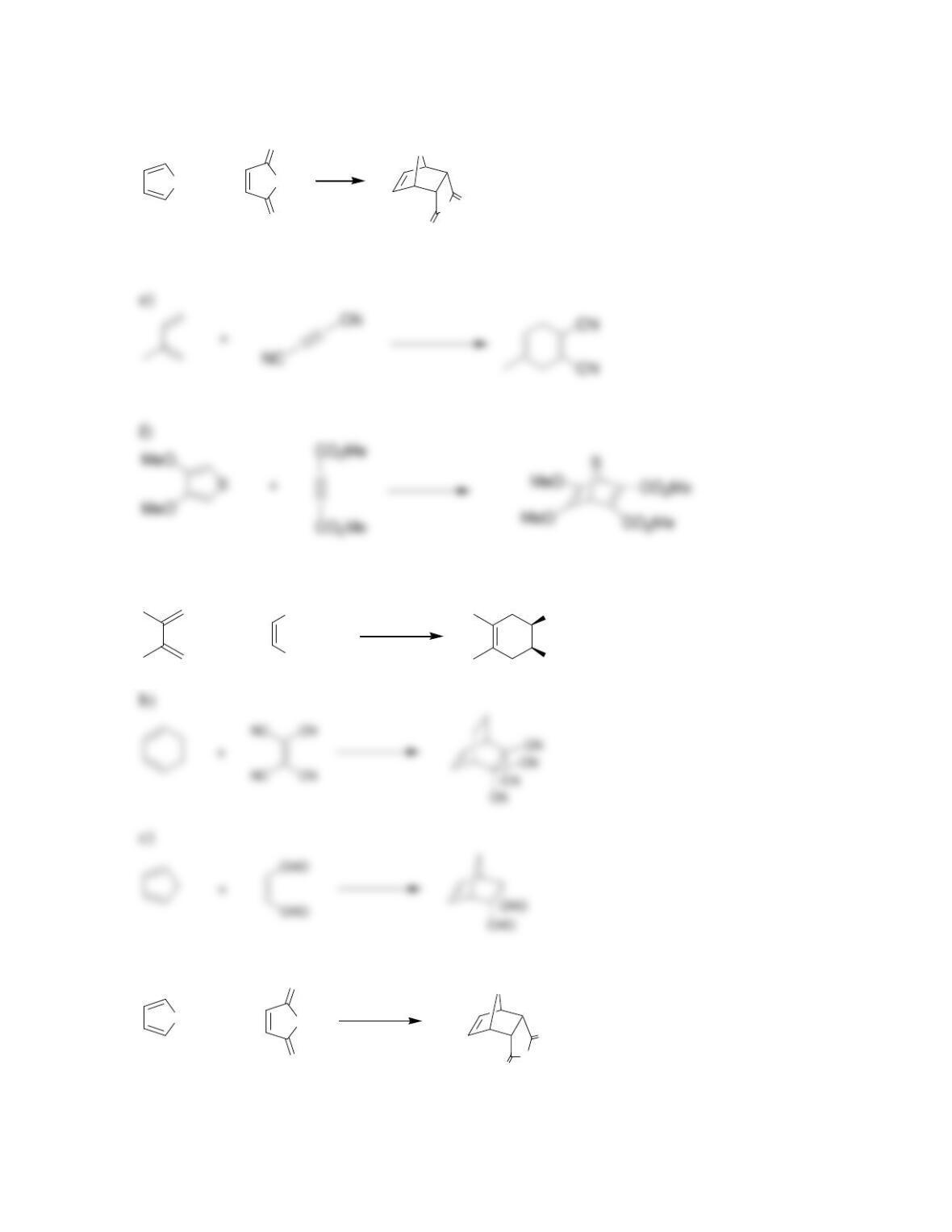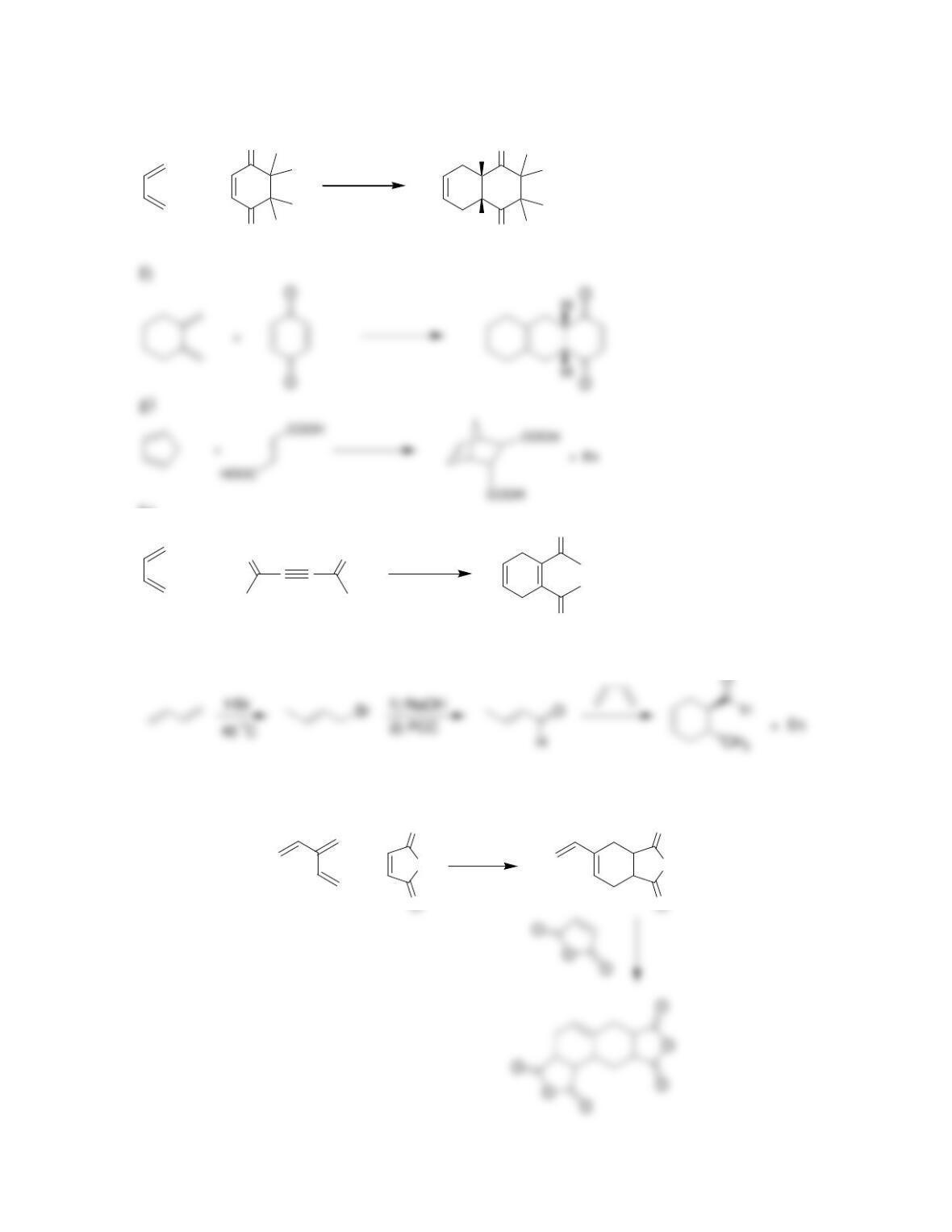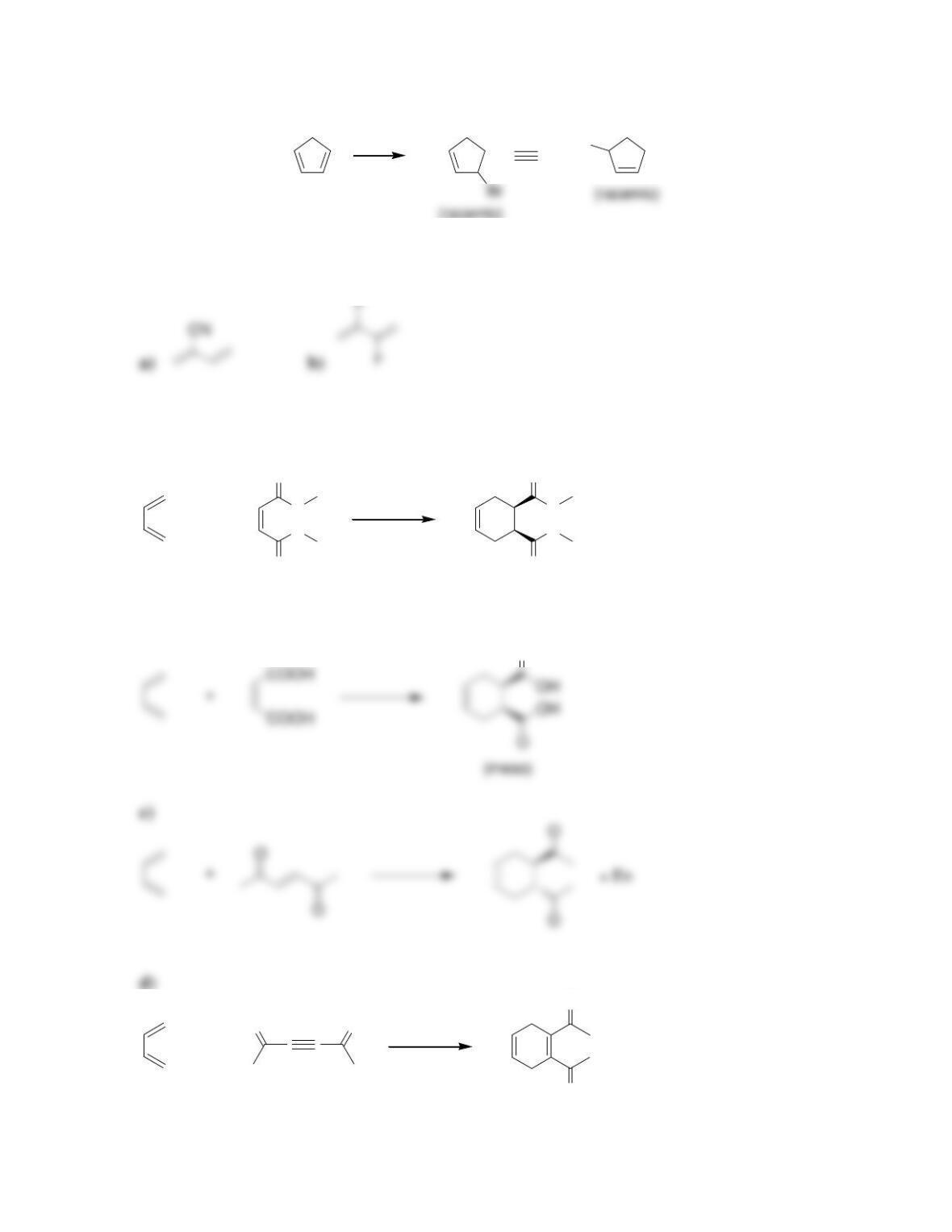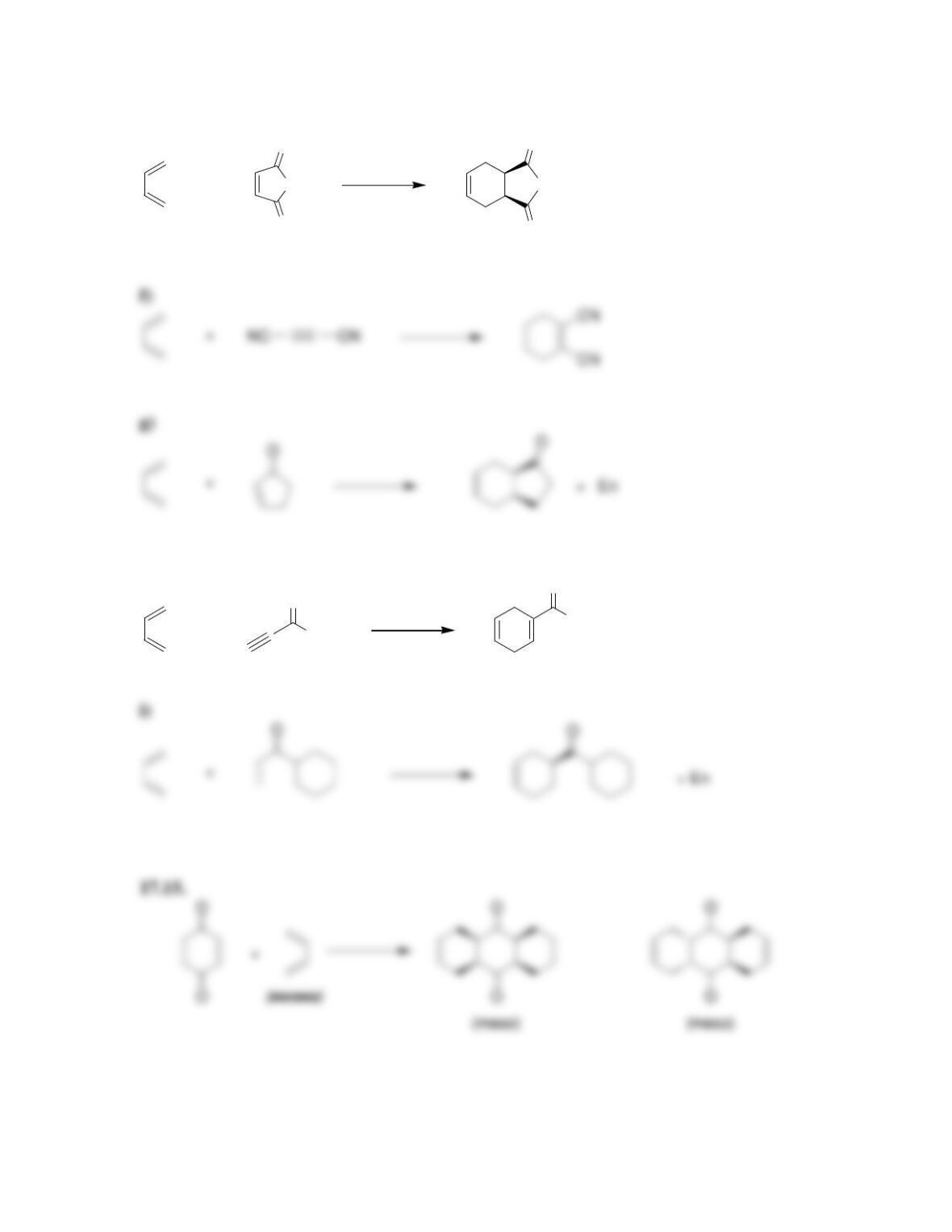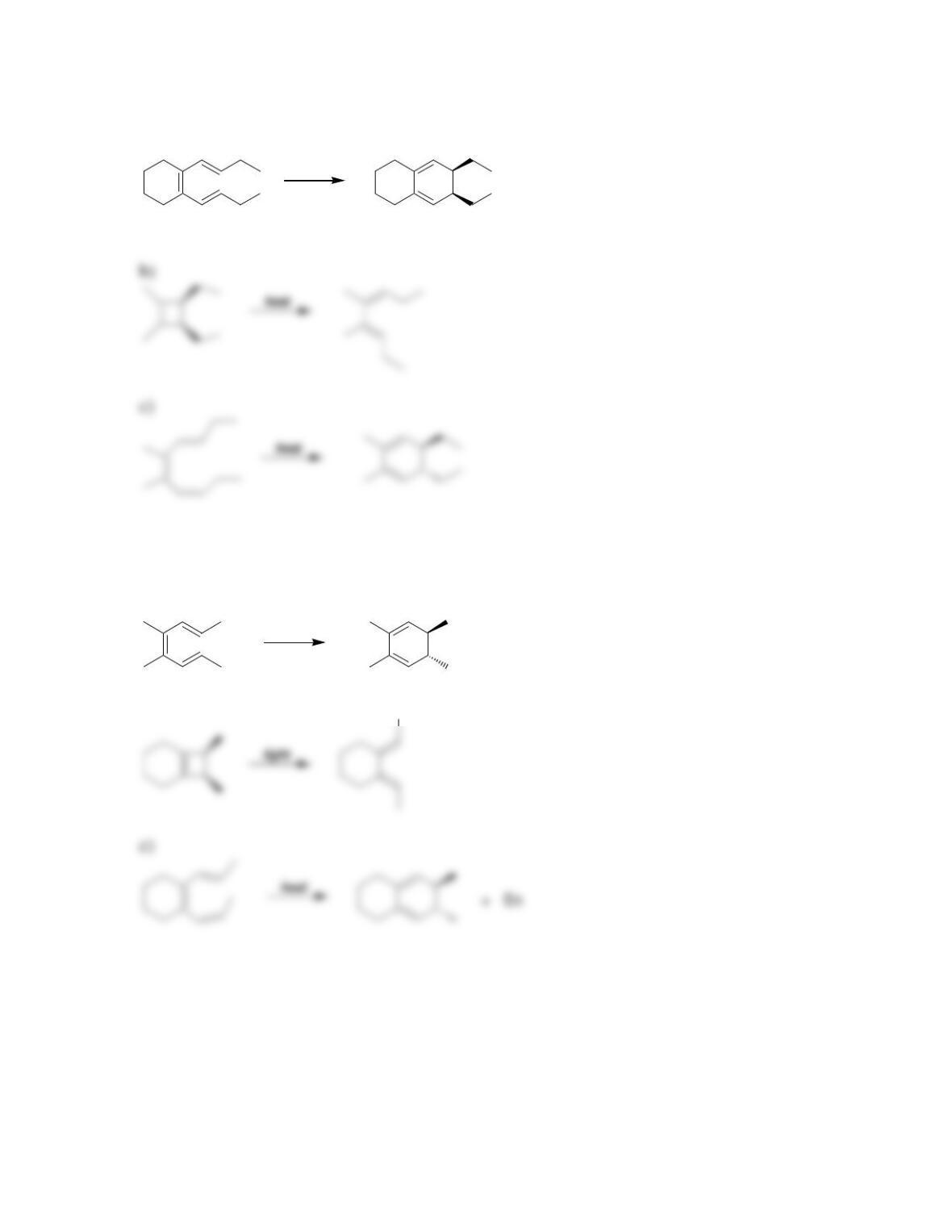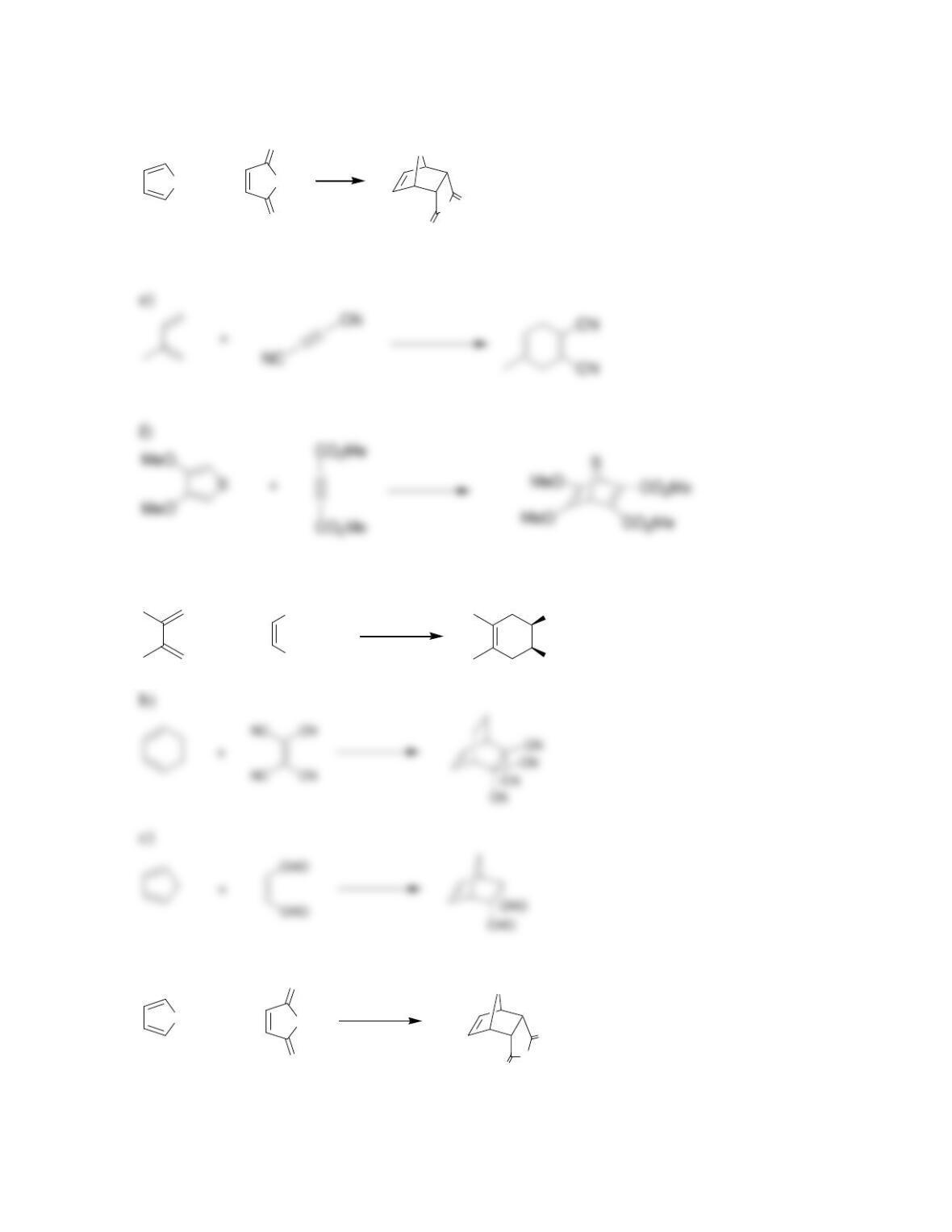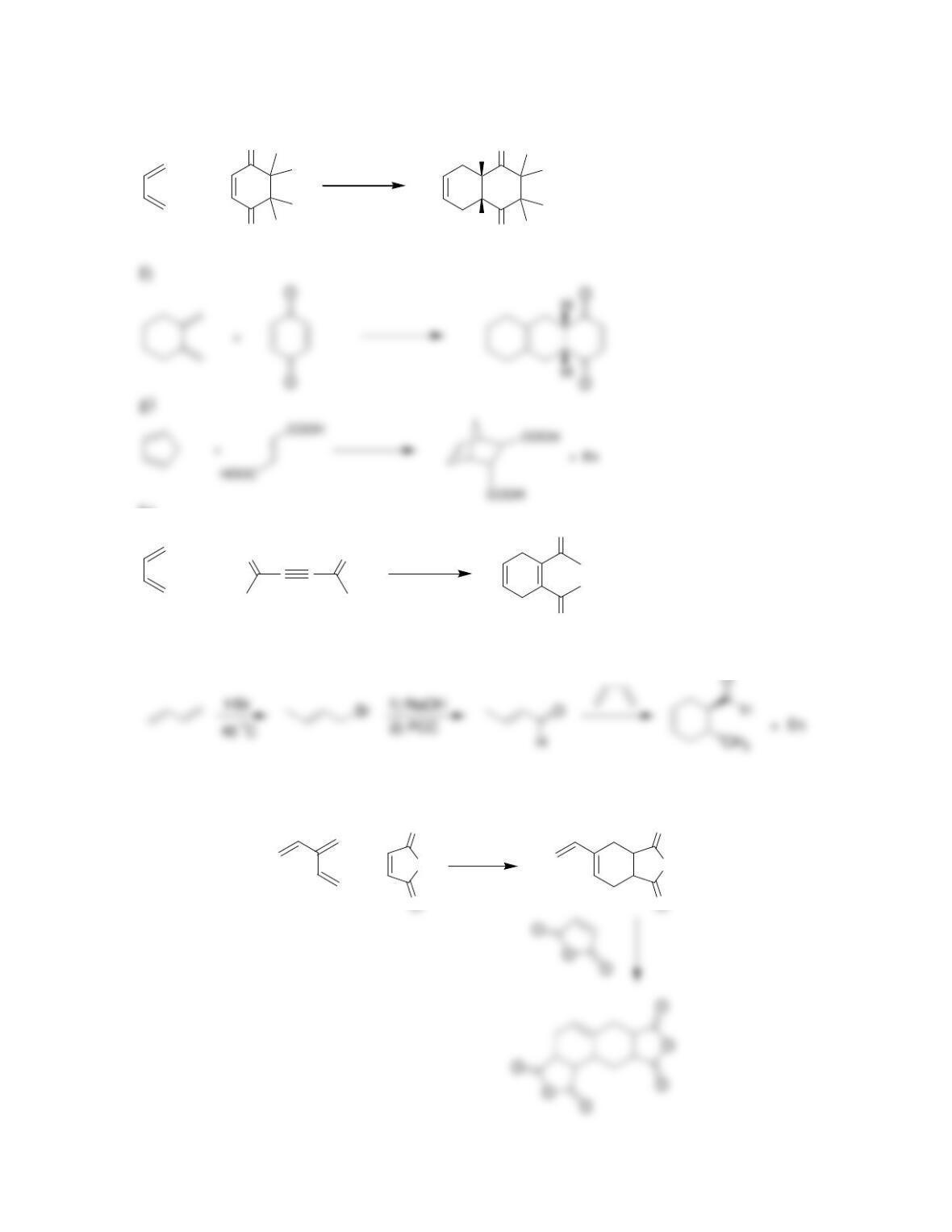Chapter 17
Conjugated Pi Systems and Pericyclic Reactions
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 17. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• Reactions induced by light are called _______________ reactions.
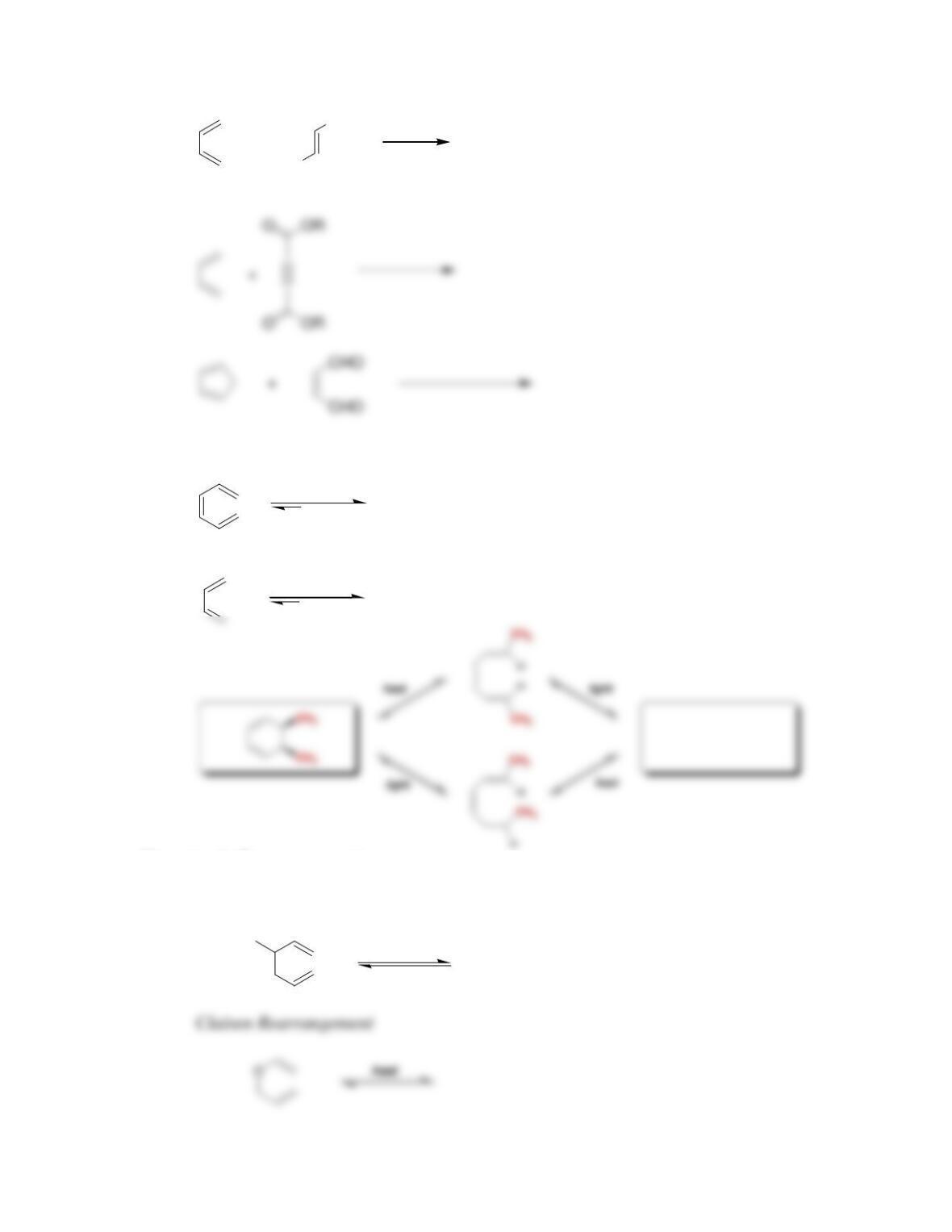
• When butadiene is treated with HBr, two major products are observed, resulting
from ______-addition and ______-addition.
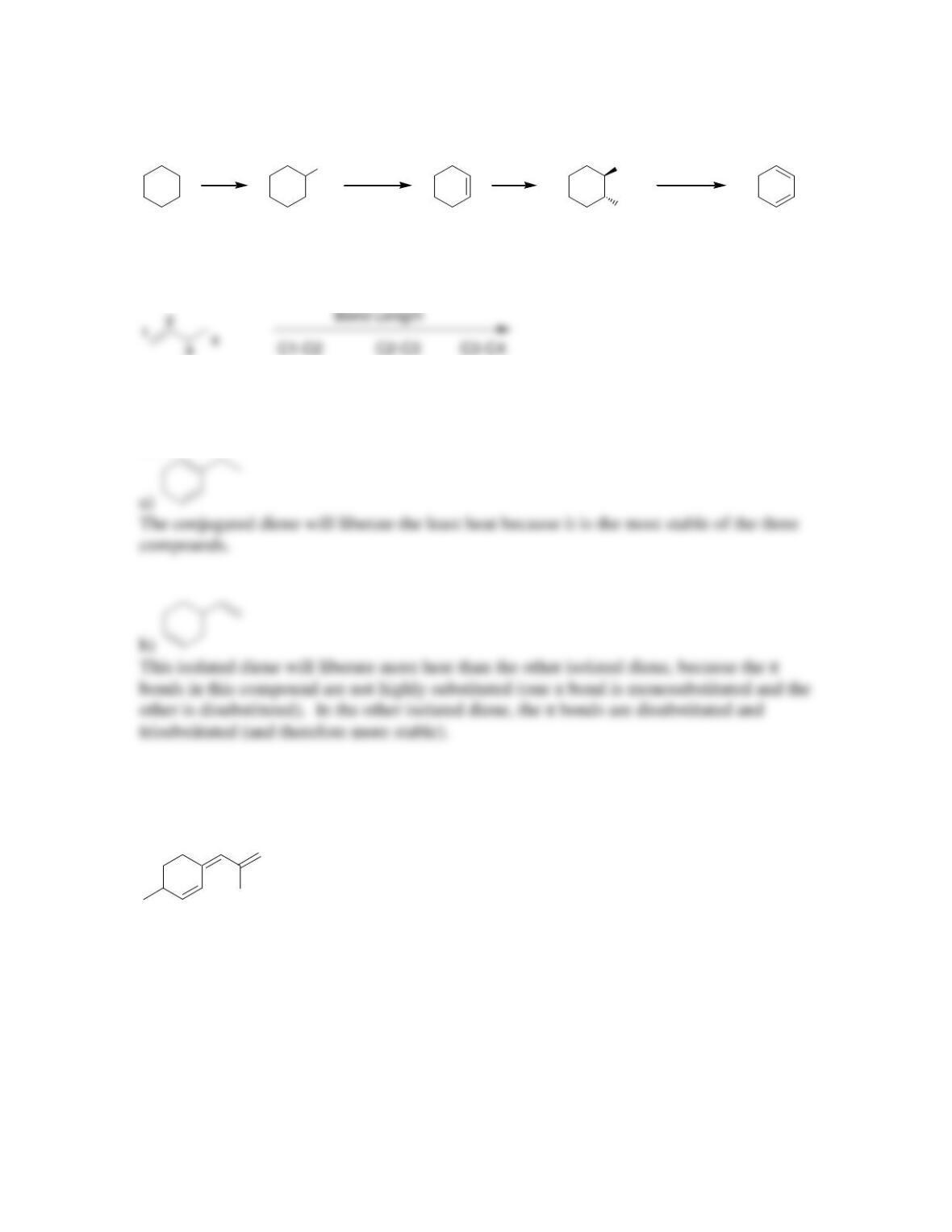
• Conjugated dienes that undergo addition at low temperature are said to be under
____________ control. Conjugated dienes that undergo addition at elevated
temperature are said to be under _________________ control.
is obtained, and the _____ cycloadduct is favored over the _____ cycloadduct.
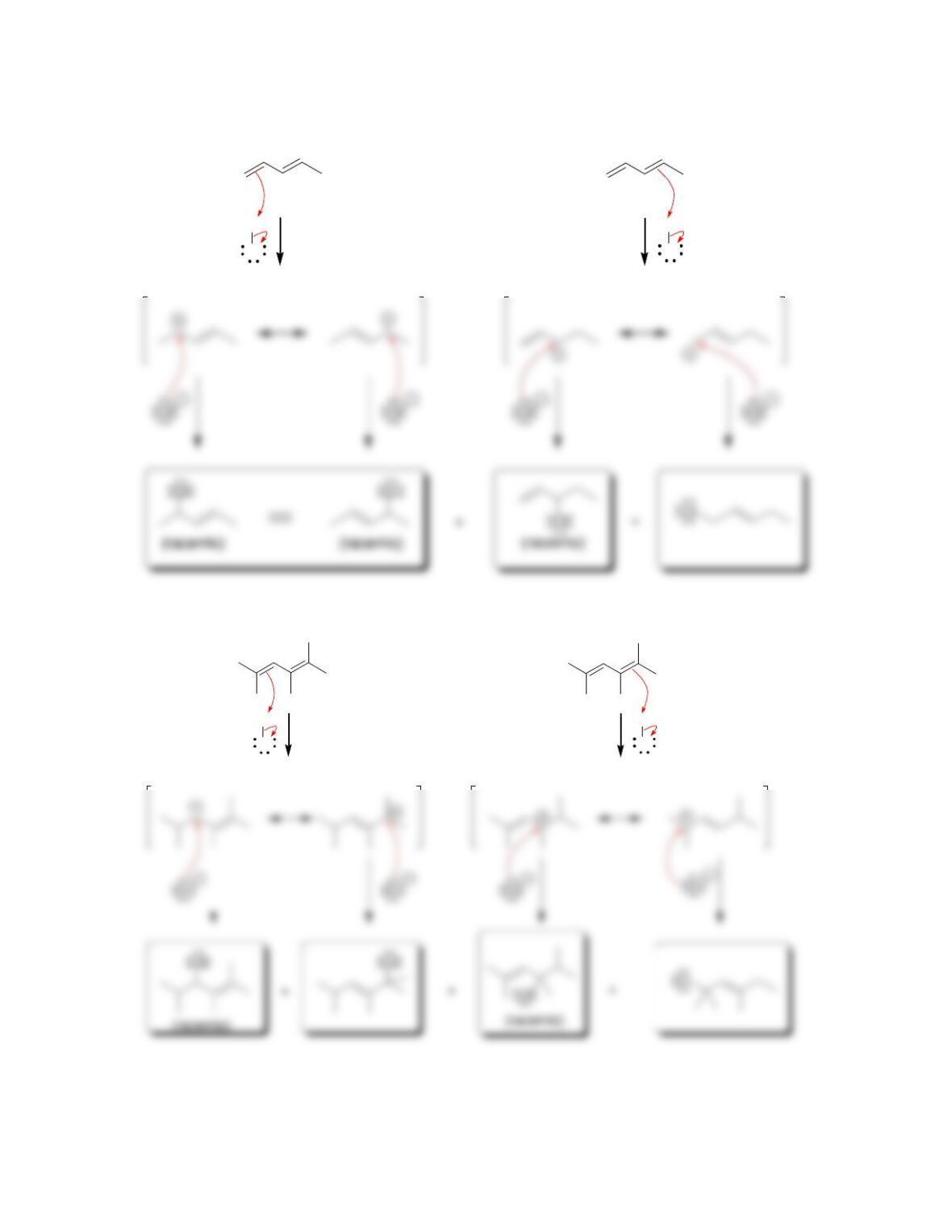
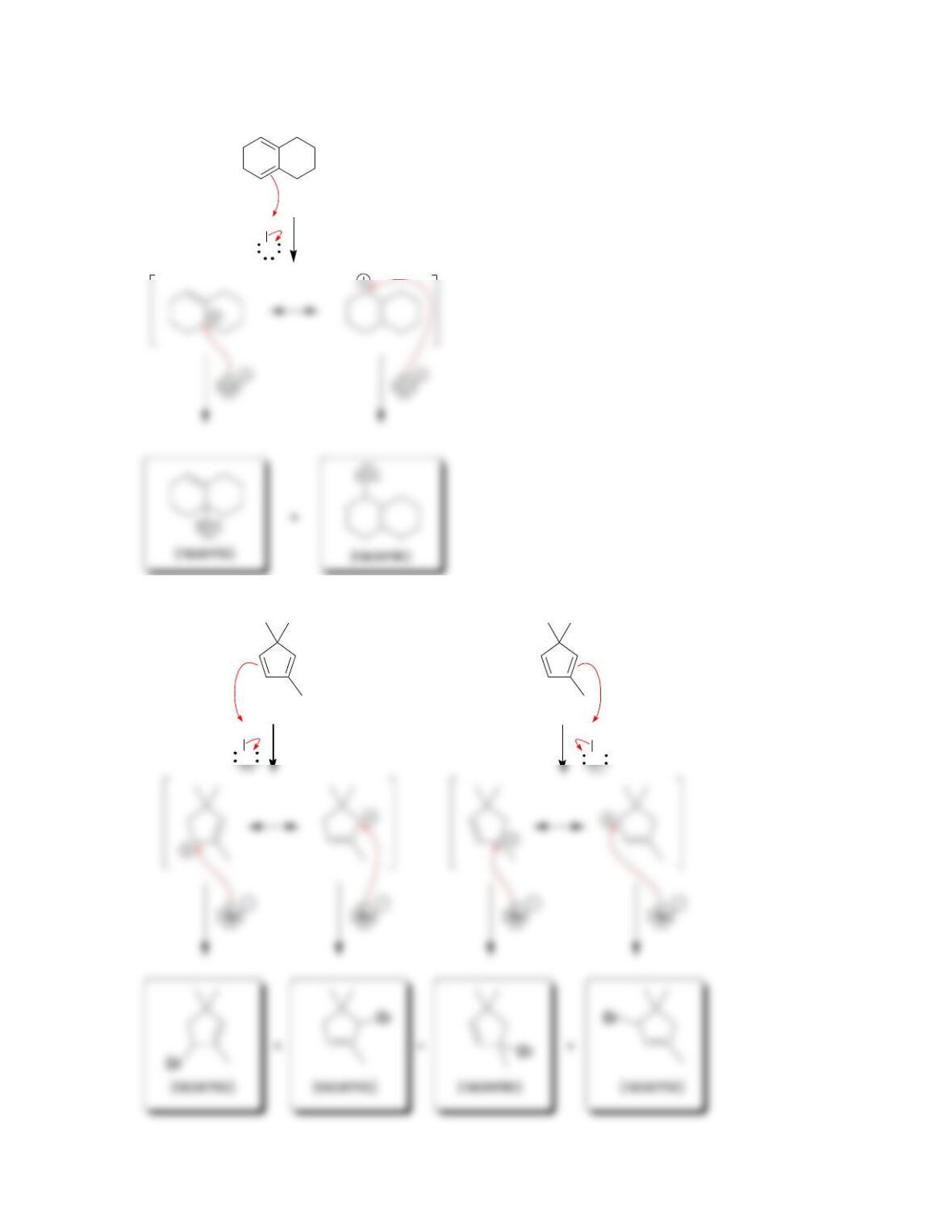
• Conservation of orbital symmetry determines whether an electrocyclic reaction
occurs in a ___________ fashion or a ____________ fashion.
• A [ _________ ] sigmatropic rearrangement is called a Cope rearrangement
when all six atoms of the cyclic transition state are carbon atoms.