Chapter 15
Infrared Spectroscopy and Mass Spectrometry
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 15. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• Spectroscopy is the study of the interaction between _______ and ________.
• The difference in energy (∆E) between vibrational energy levels is determined
by the nature of the bond. If a photon of light possesses exactly this amount
of energy, the bond can absorb the photon to promote a __________________
excitation.
• IR spectroscopy can be used to identify which _____________________ are
present in a compound.
• The location of each signal in an IR spectrum is reported in terms of a
high energy _______________, generating a radical cation that is symbolized
by (M)
+•
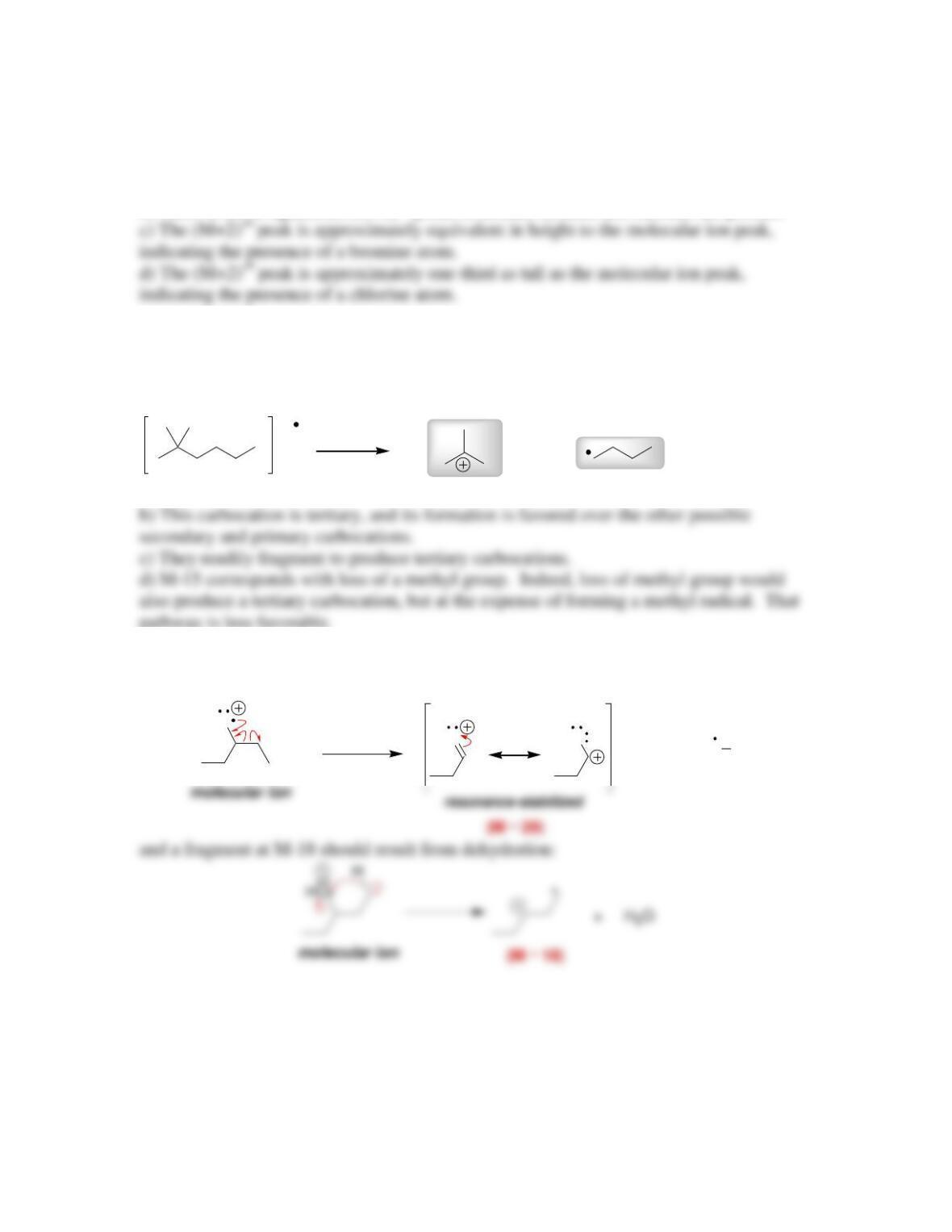
and is called the molecular ion, or the __________ ion.
• Only the molecular ion and the cationic fragments are deflected, and they are
then separated by their ____________________ (m/z).
• The tallest peak in a mass spectrum is assigned a relative value of 100% and is
called the __________ peak.
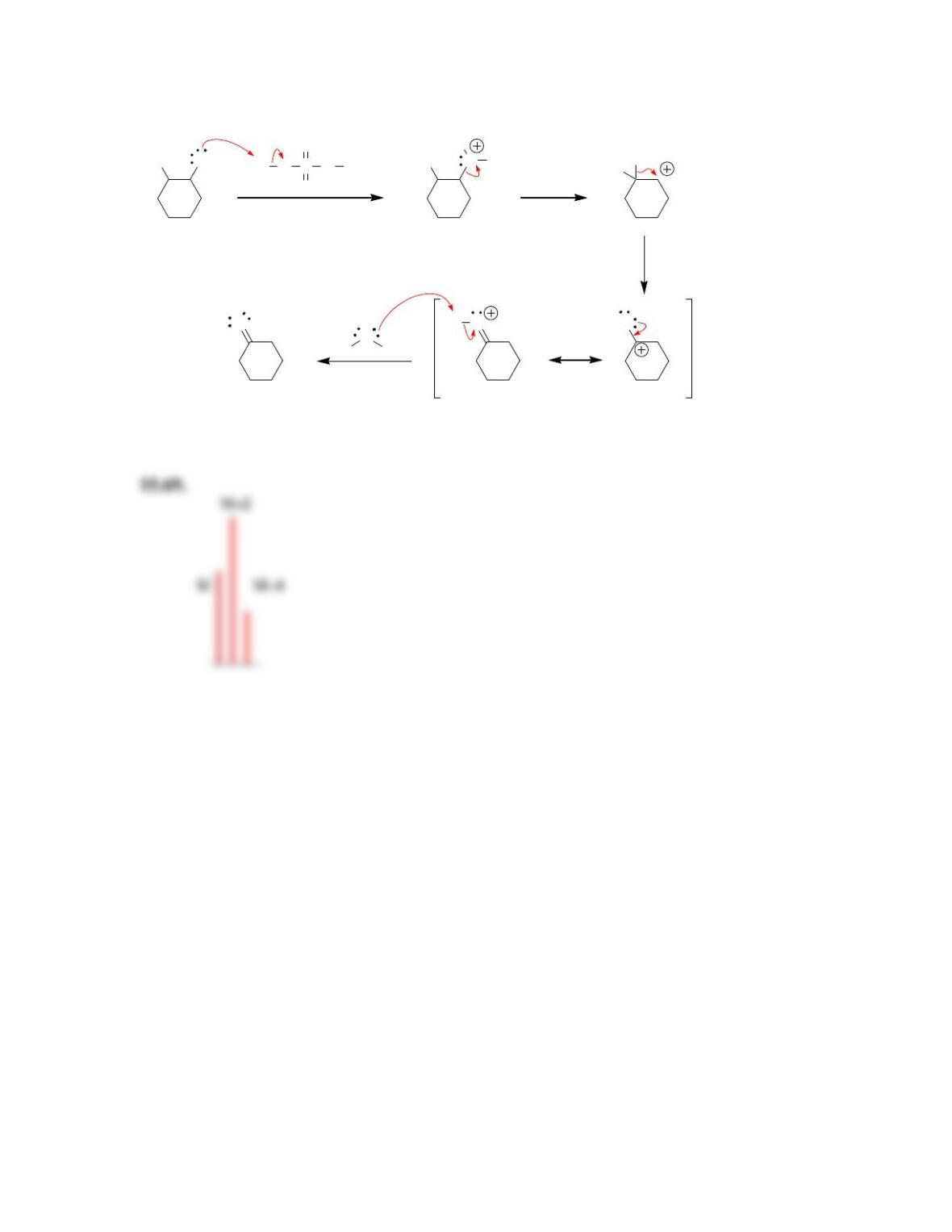
• The relative heights of the (M)
+•
peak and the (M+1)
+•
peak indicates the
number of ___________________.