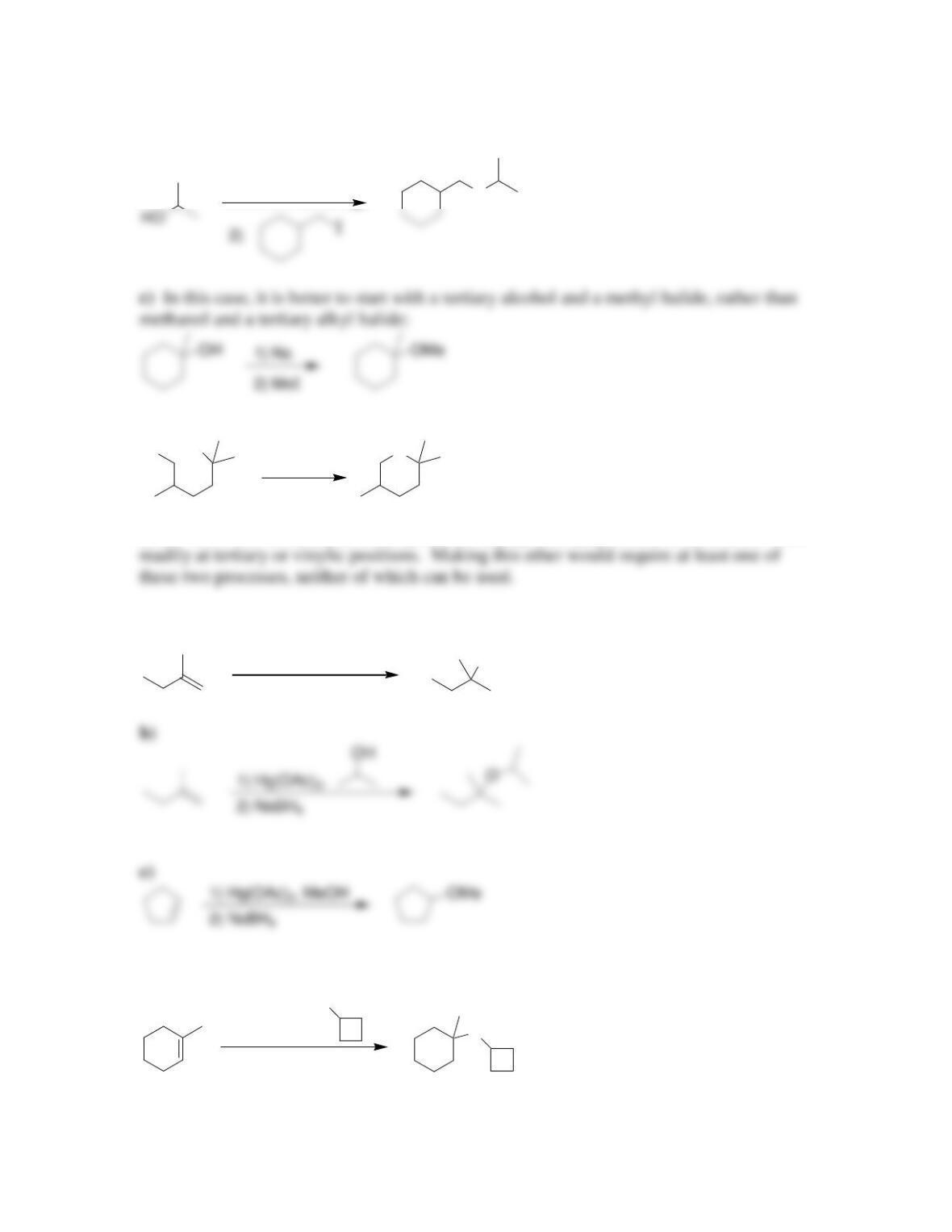
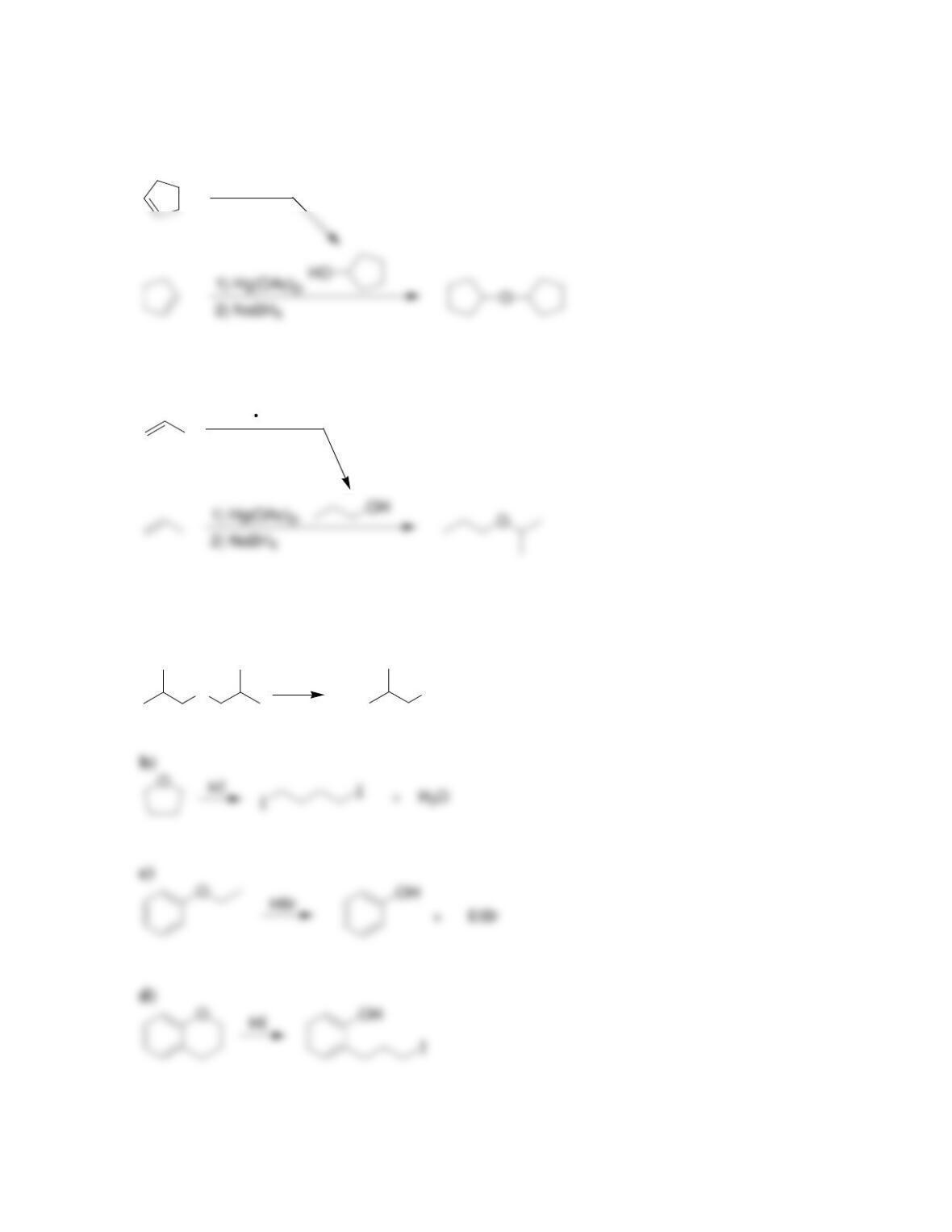
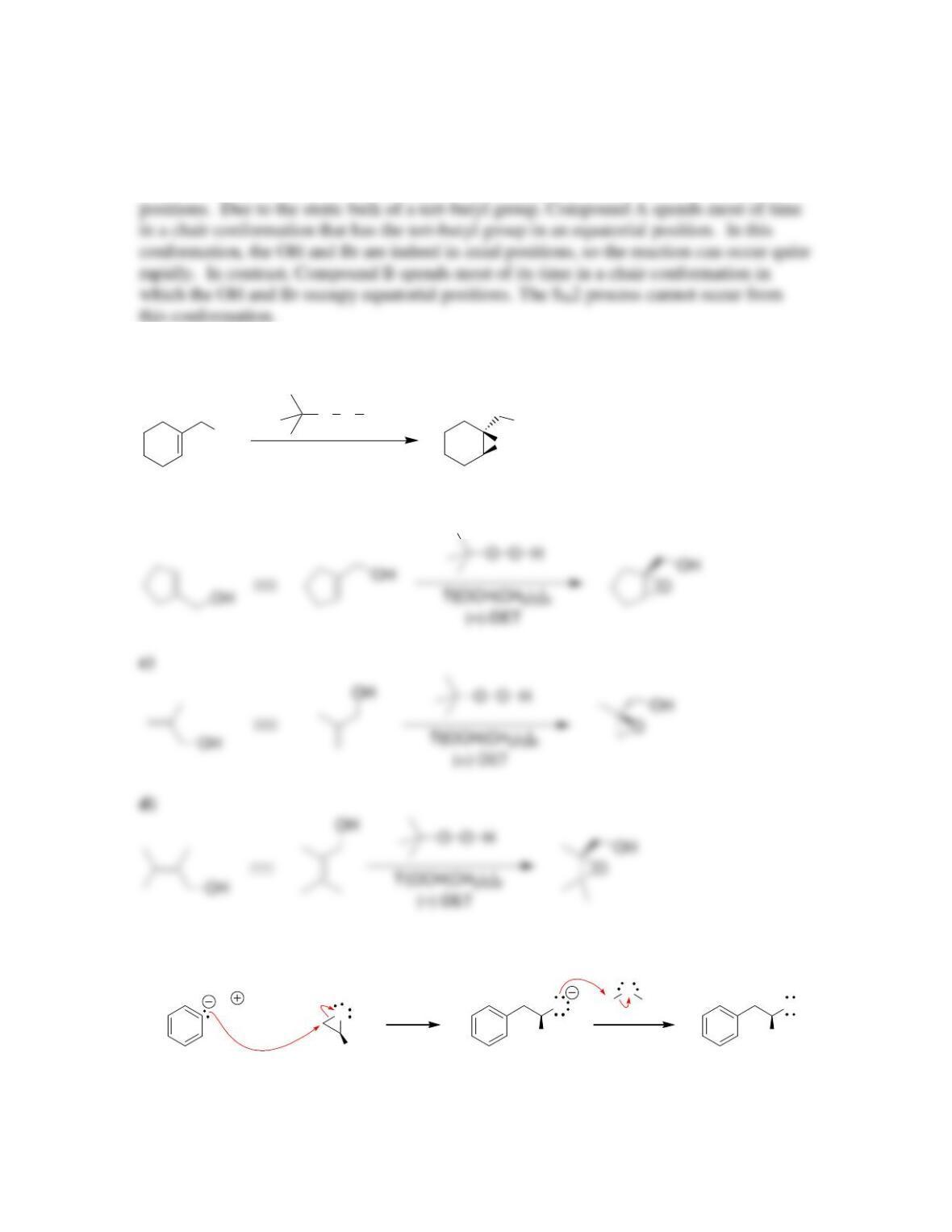
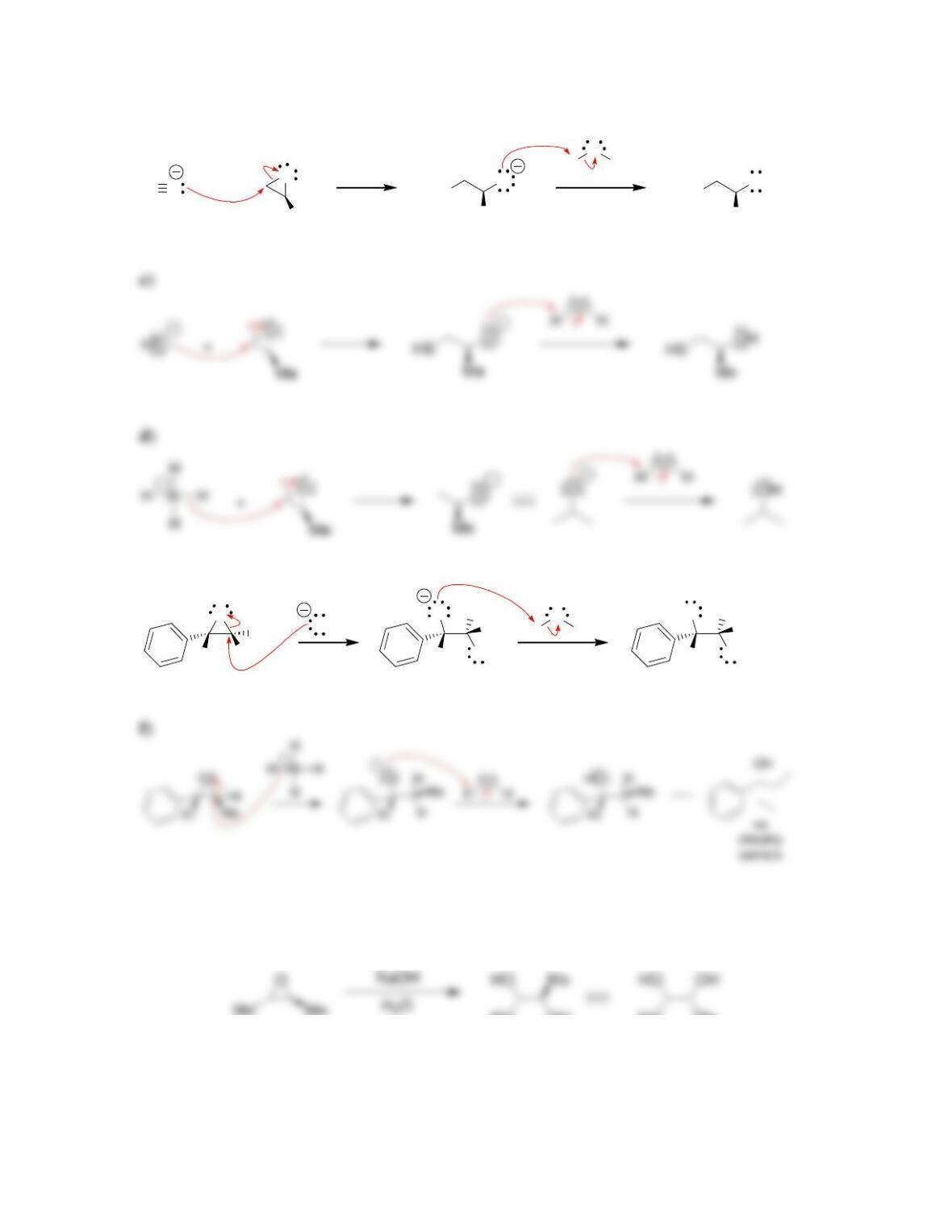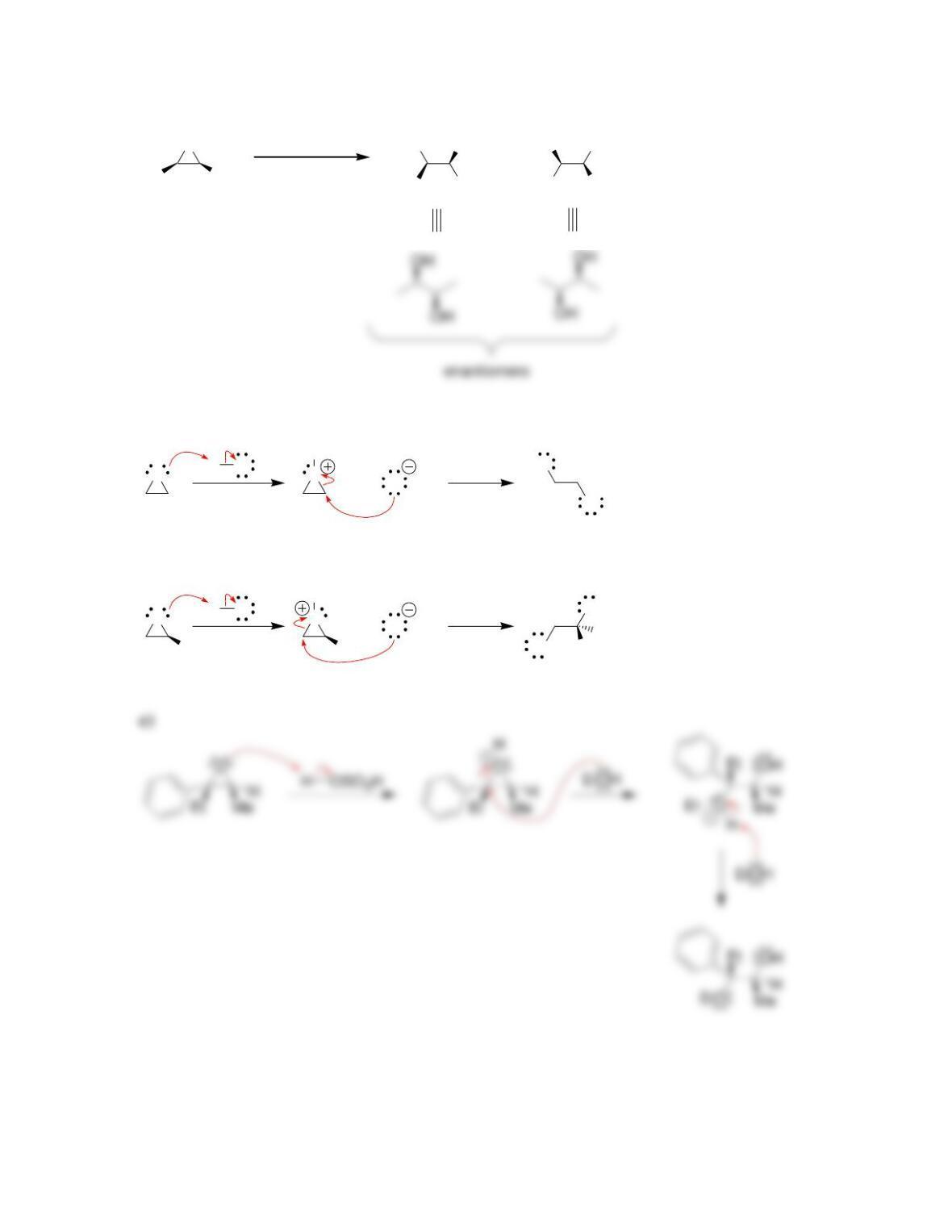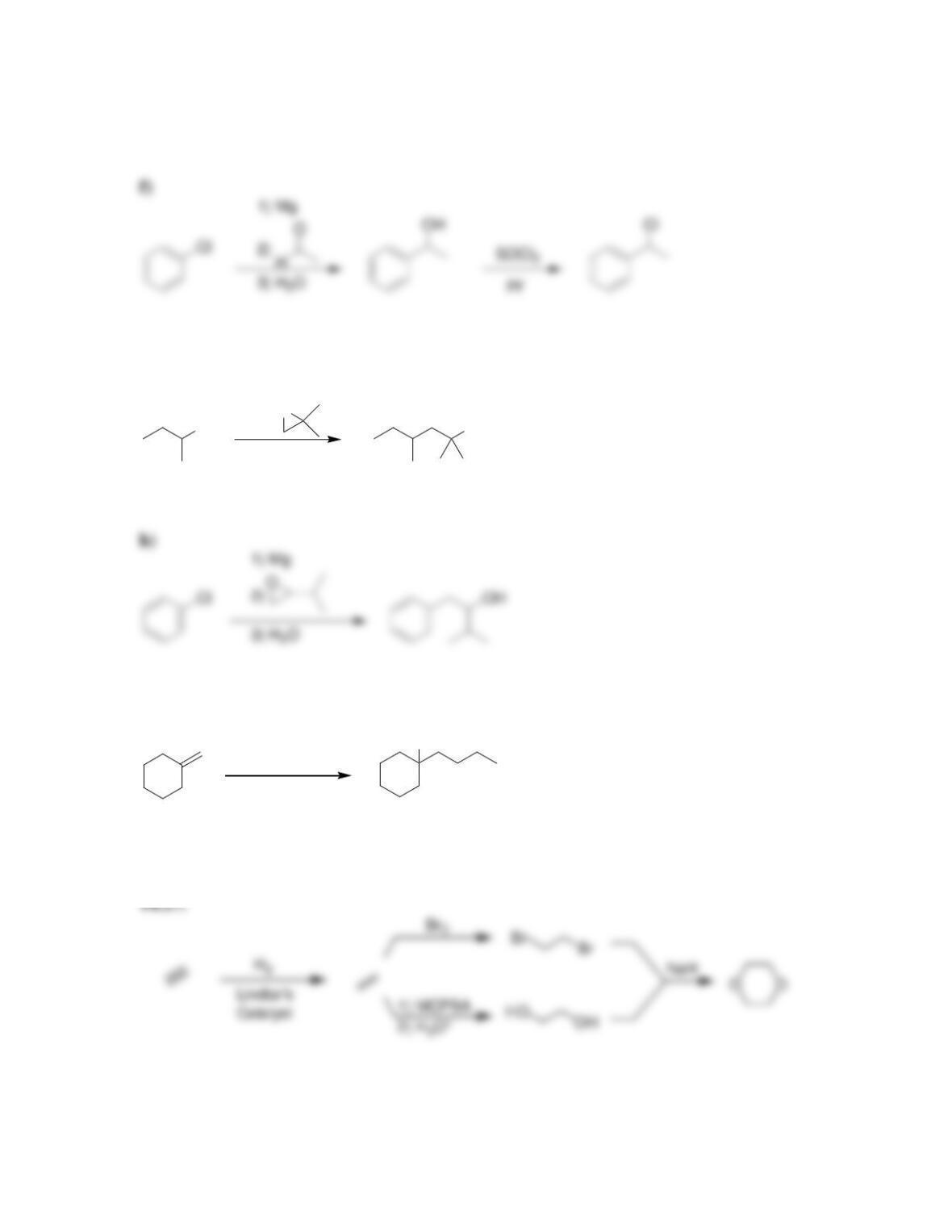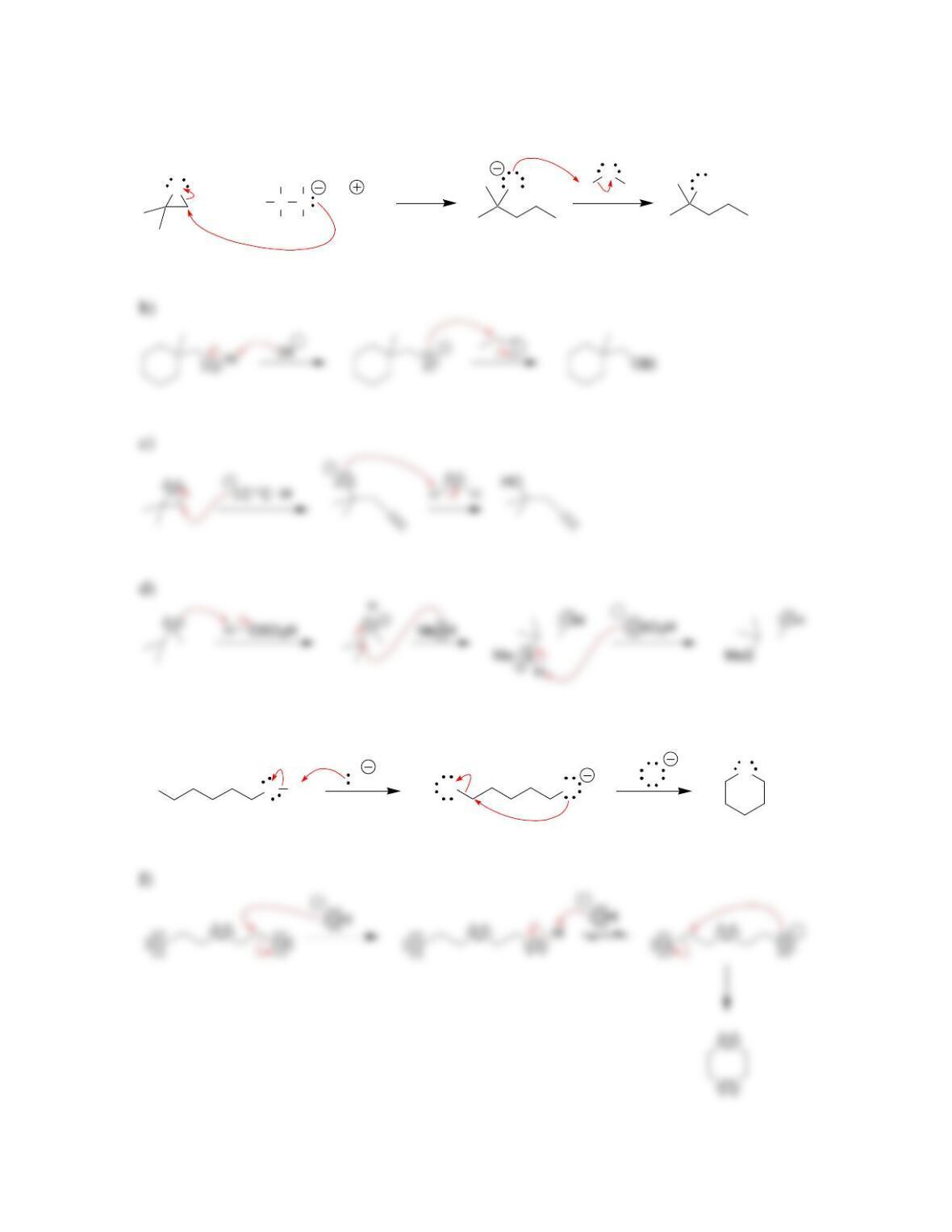Chapter 14
Ethers and Epoxides;
Thiols and Sulfides
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 14. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
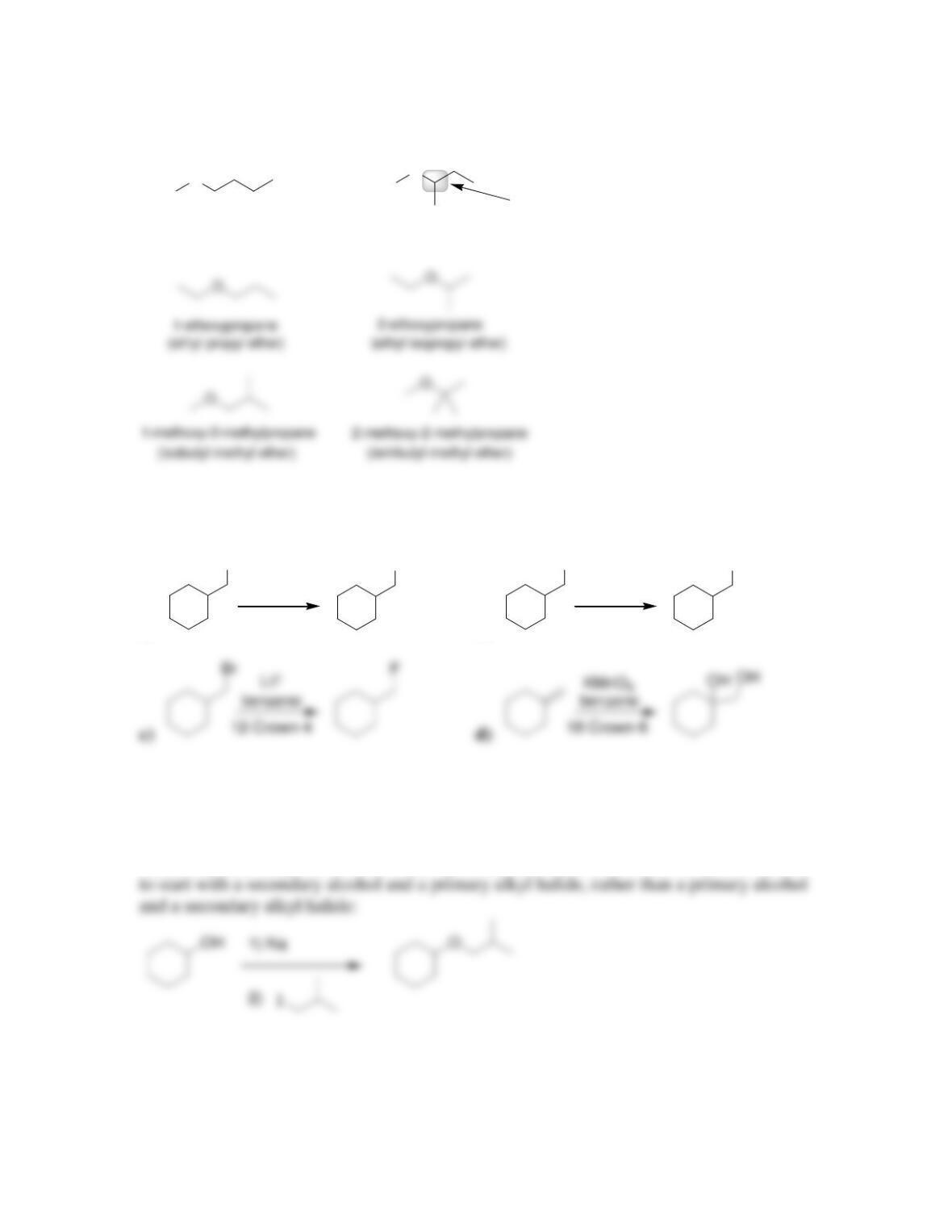
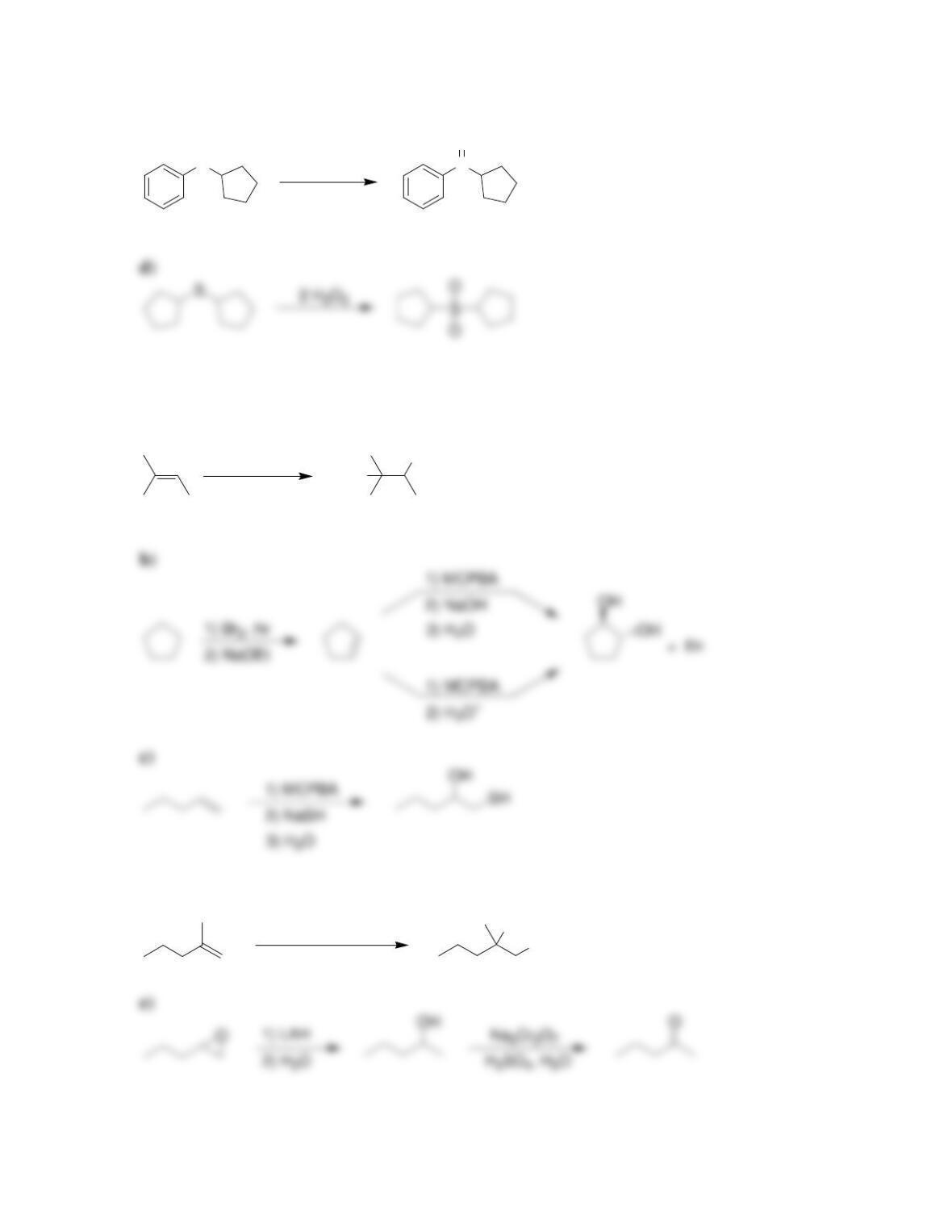
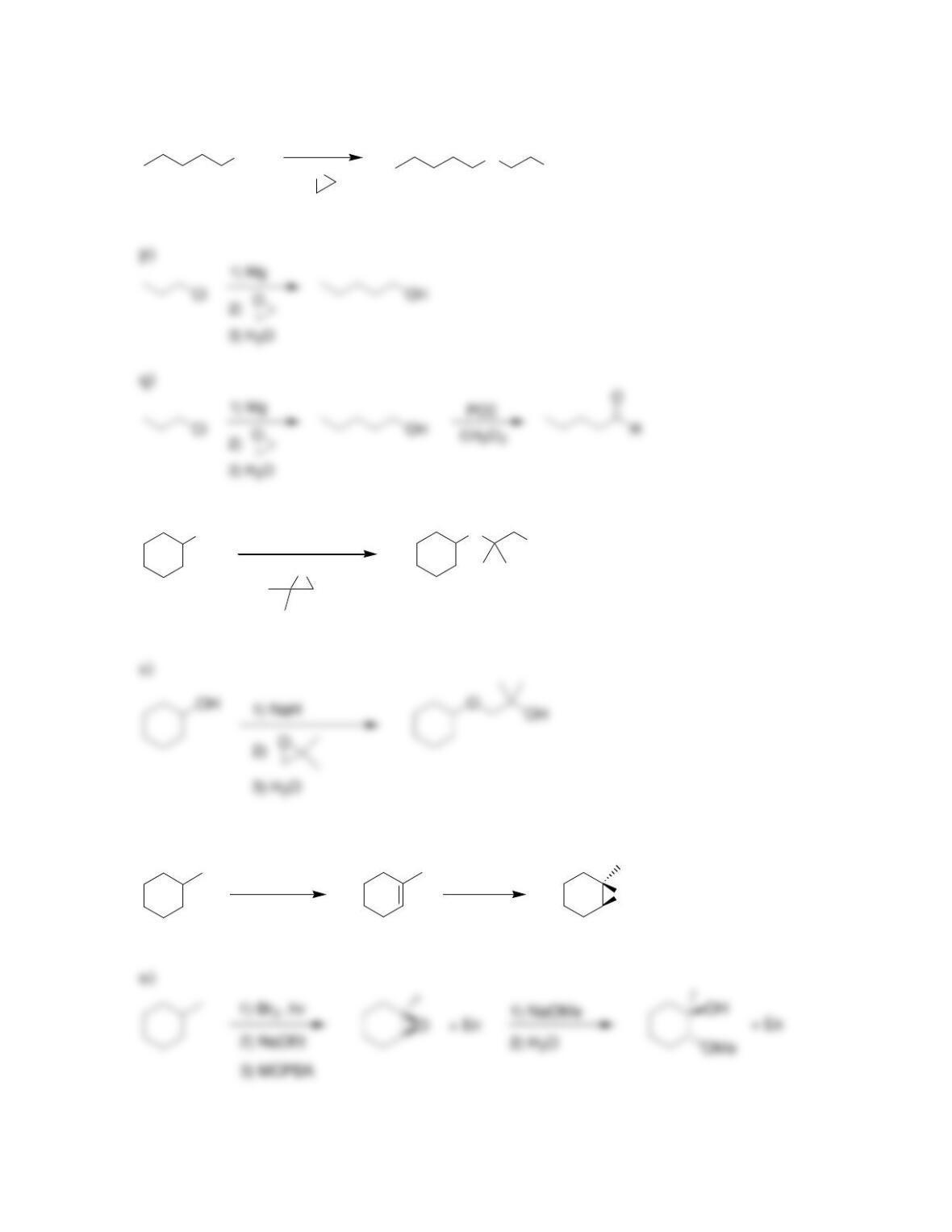
• Ethers are often used as ____________ for organic reactions.
• Cyclic polyethers, or __________ ethers, are capable of solvating metal ions in
organic (nonpolar) solvents.
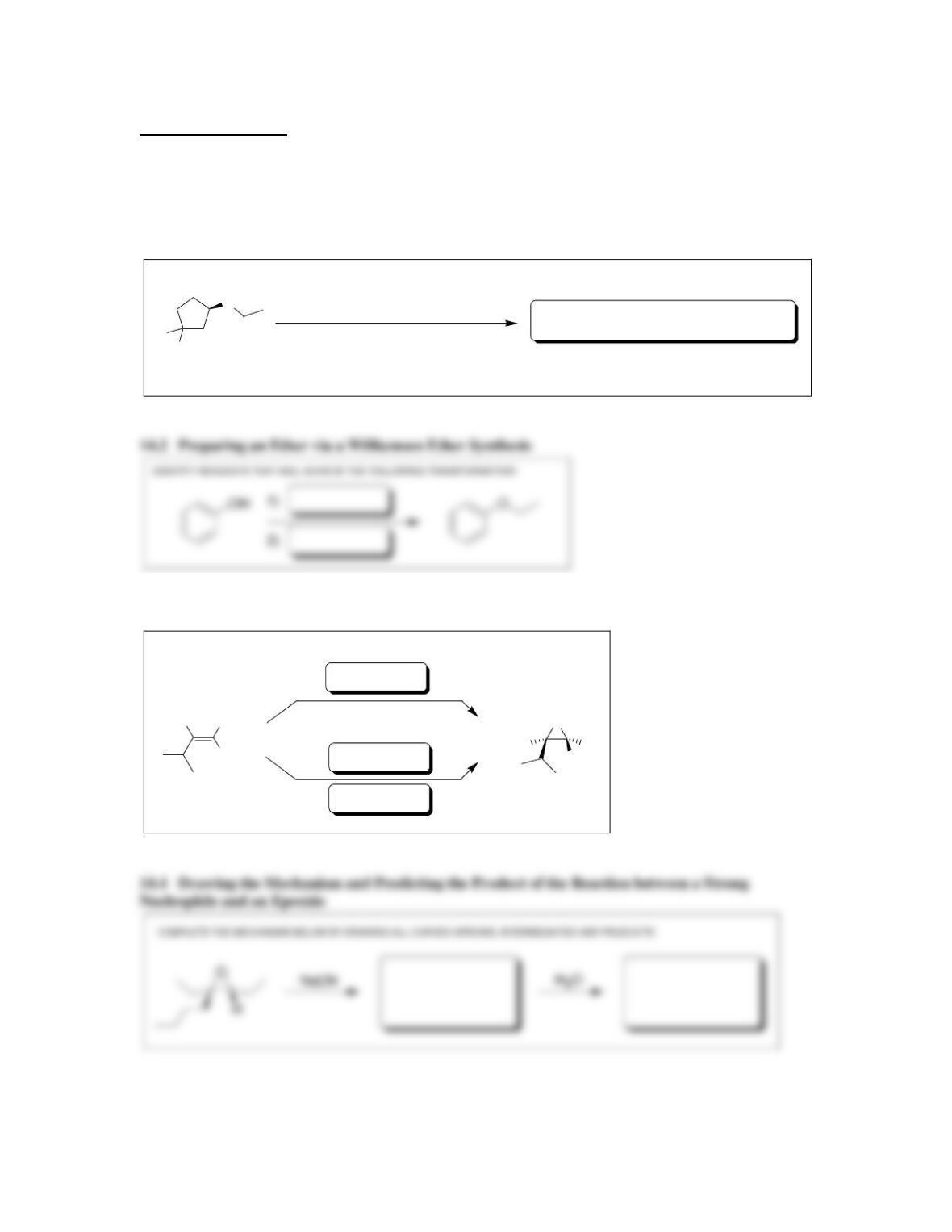
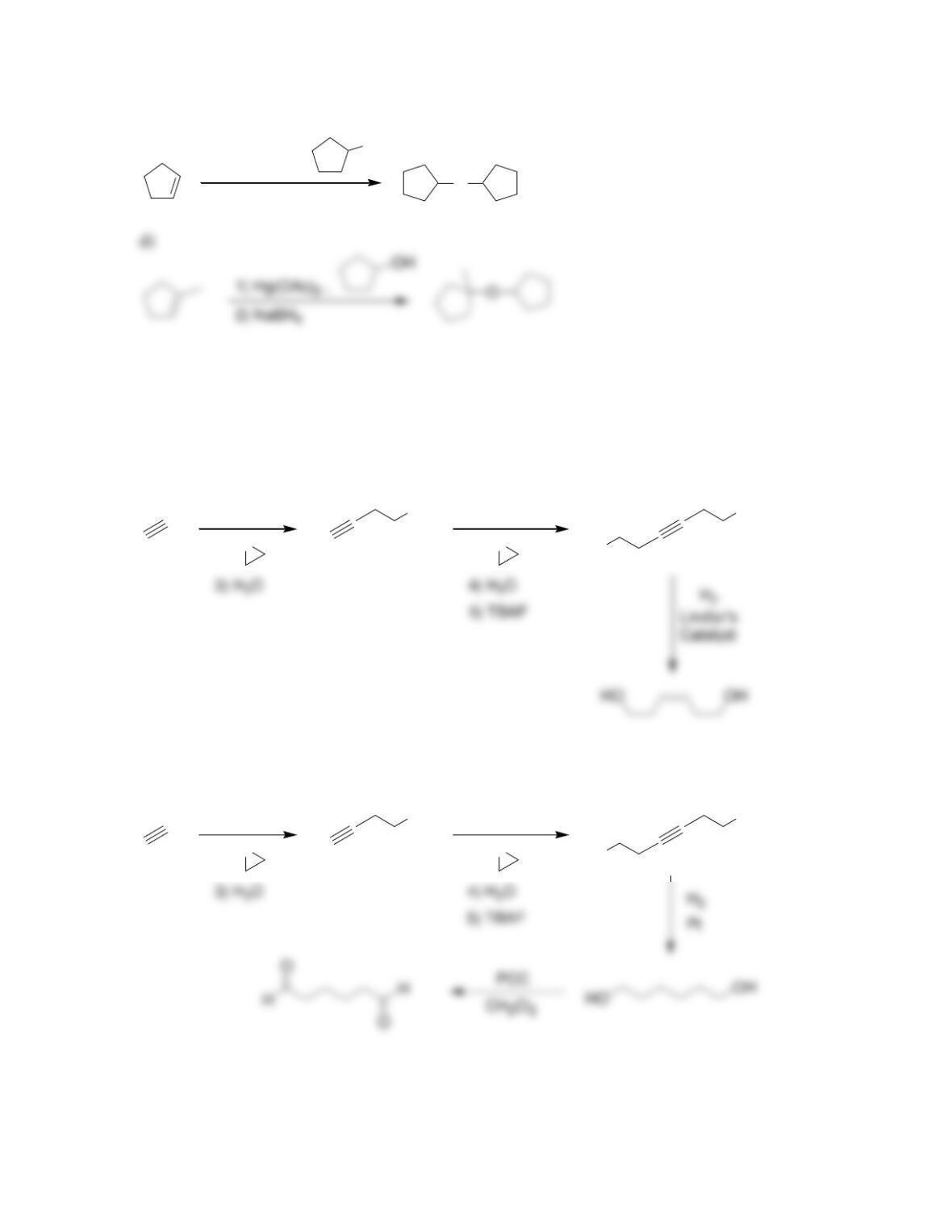
• Ethers can be readily prepared from the reaction between an alkoxide ion and an
• ______________ can be converted into epoxides by treatment with peroxy acids
or via halohydrin formation and subsequent epoxidation.
• _____________ catalysts can be used to achieve the enantioselective epoxidation
of allylic alcohols.
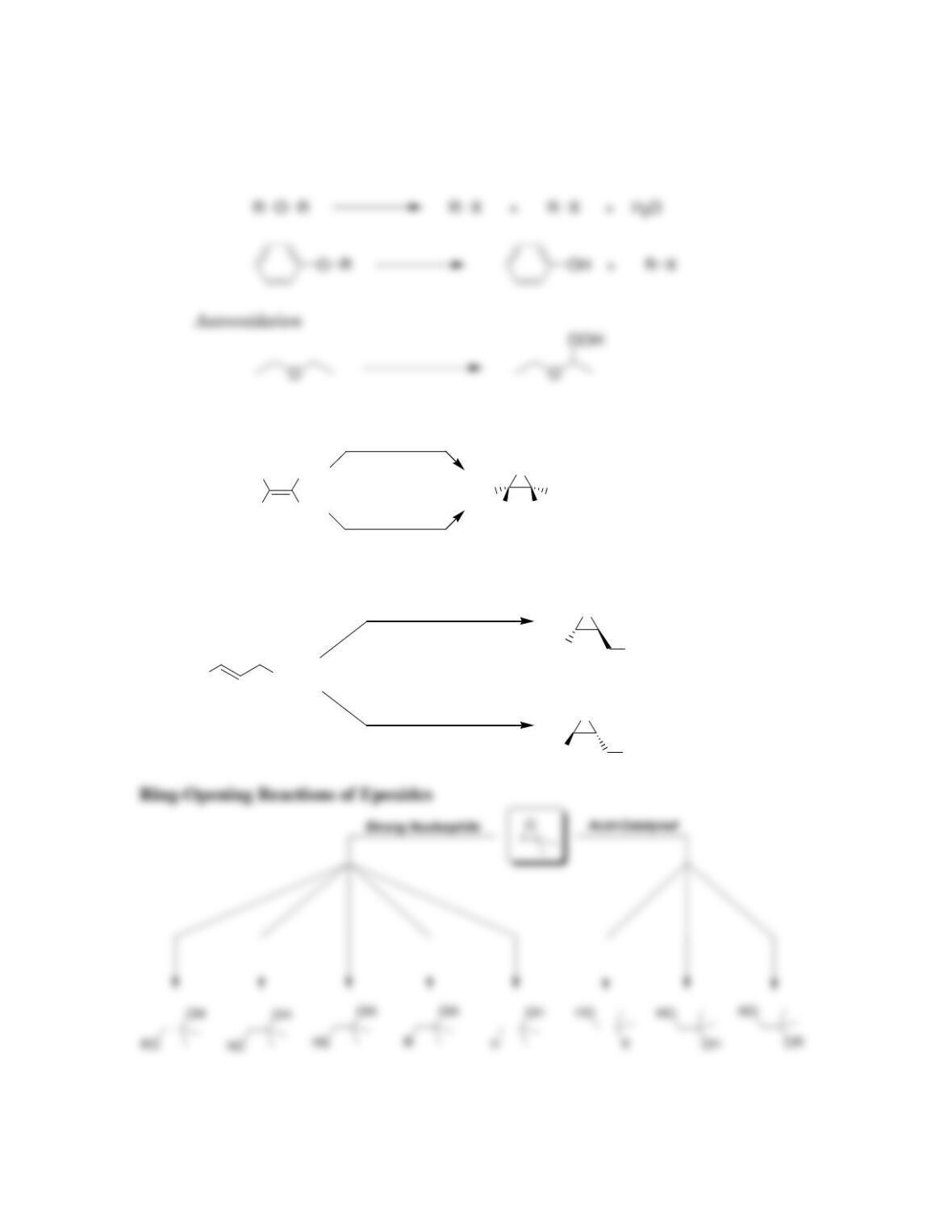
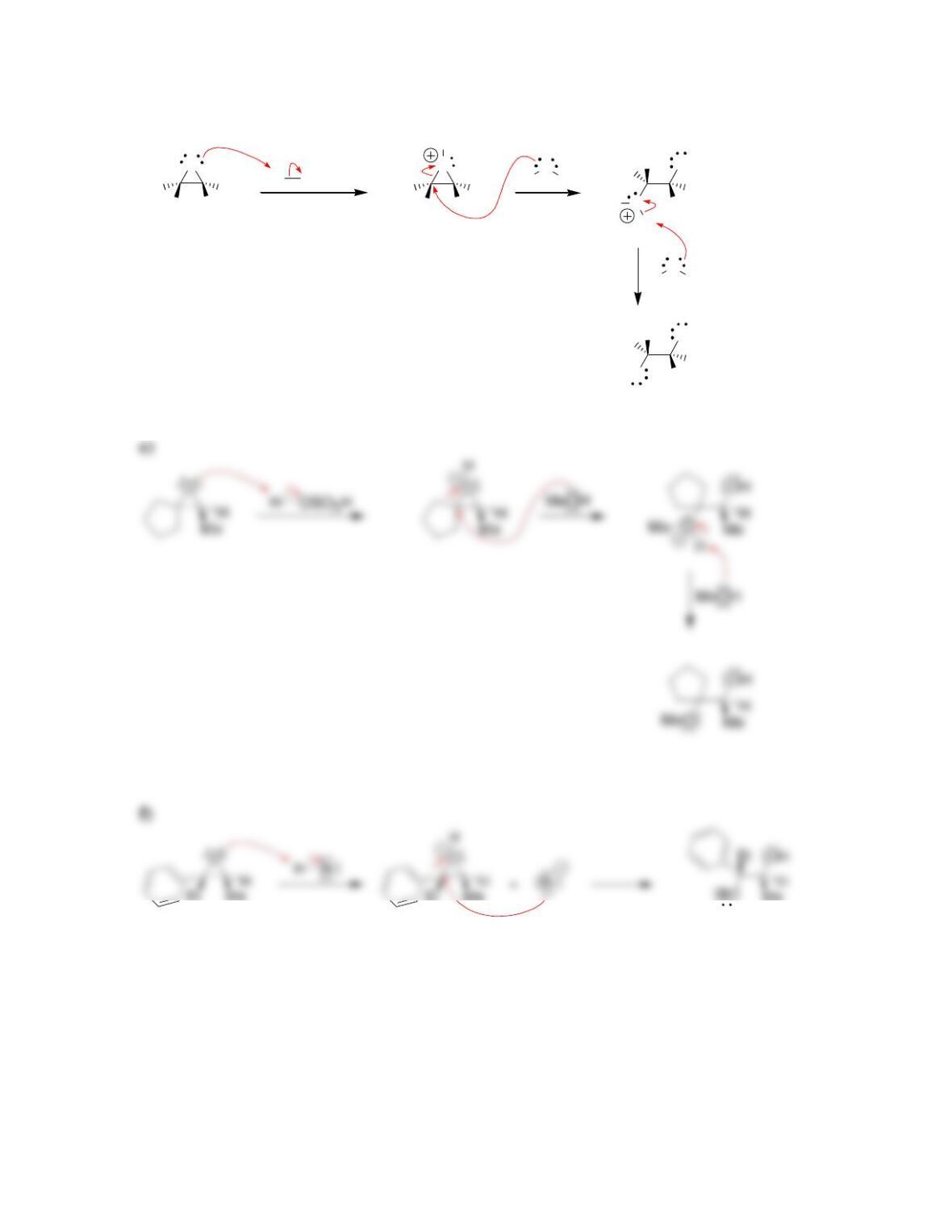
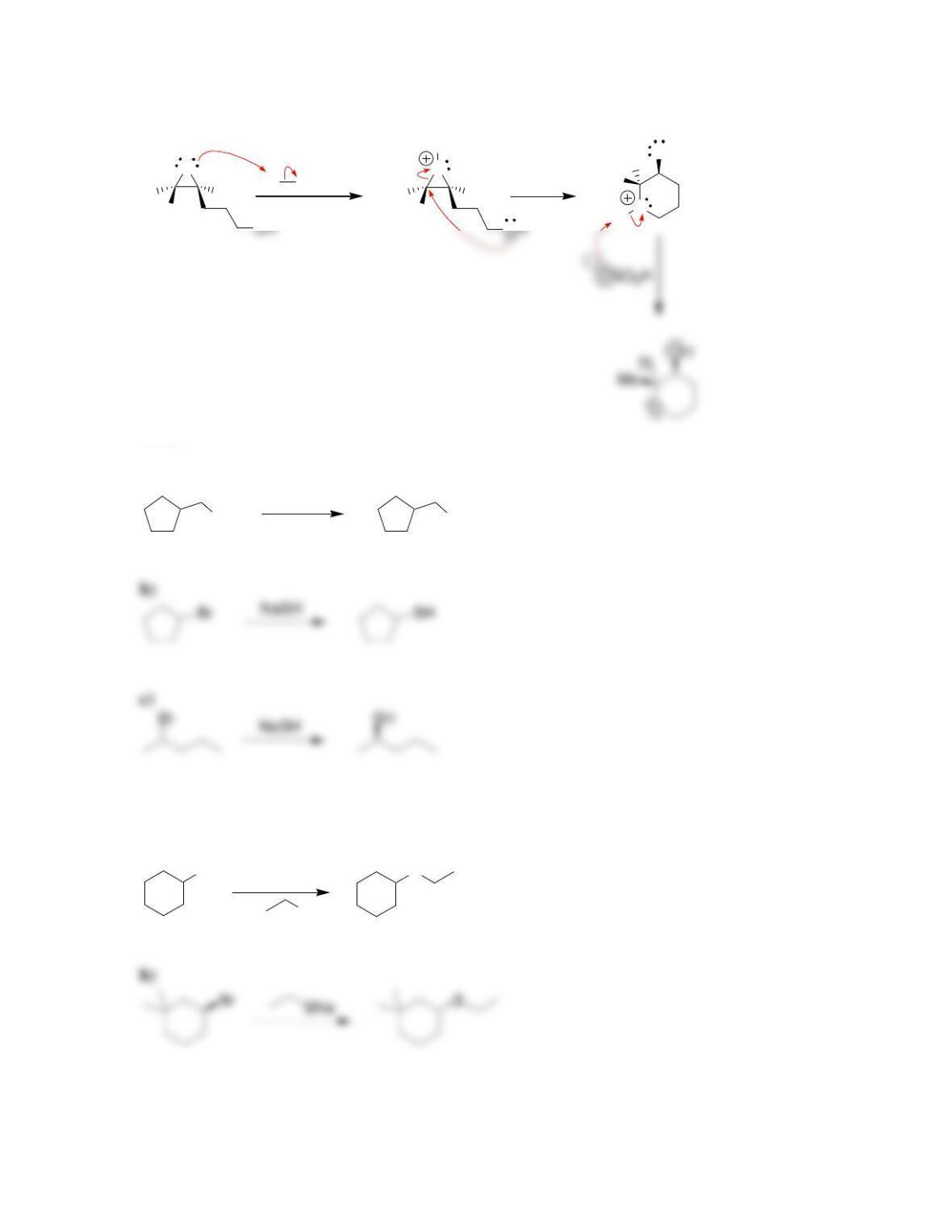
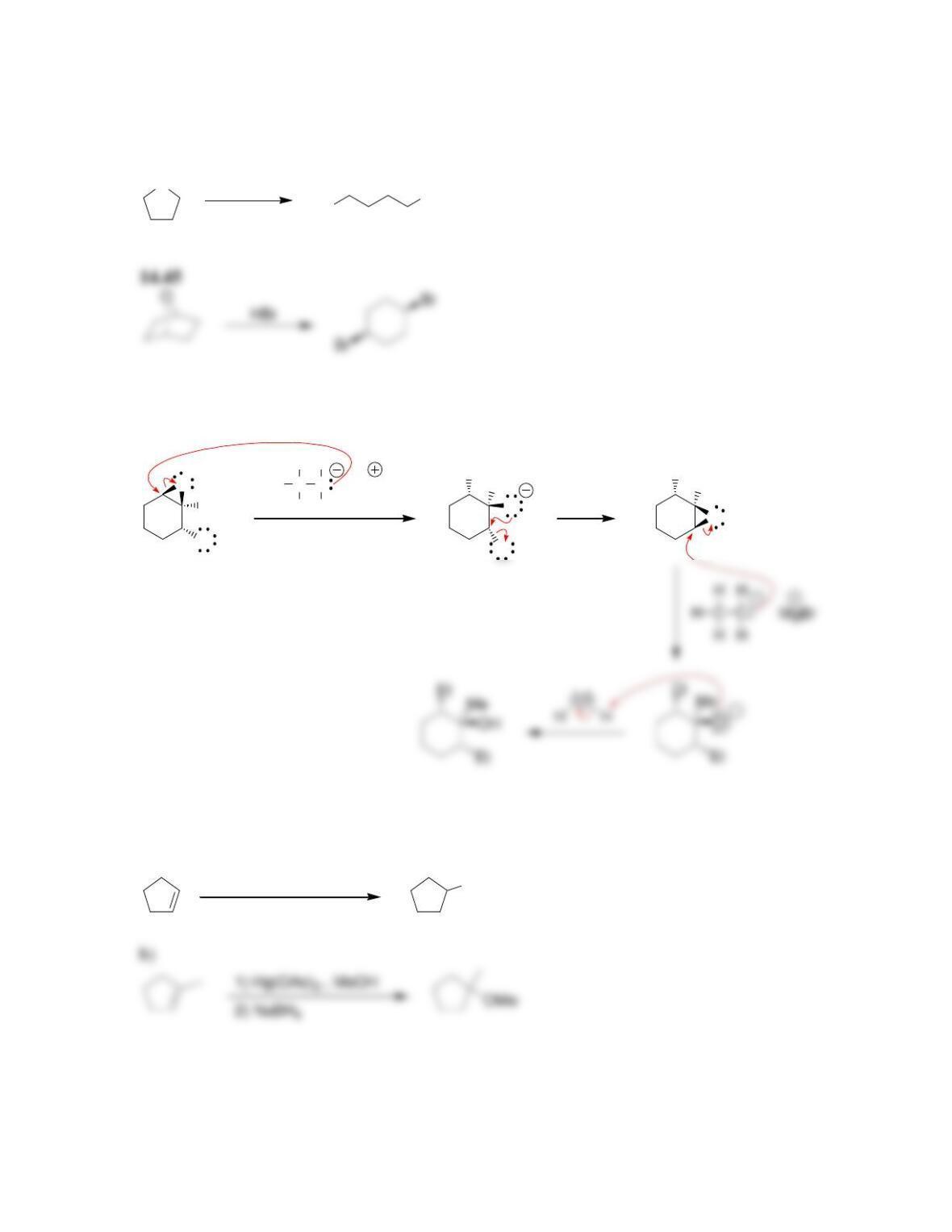
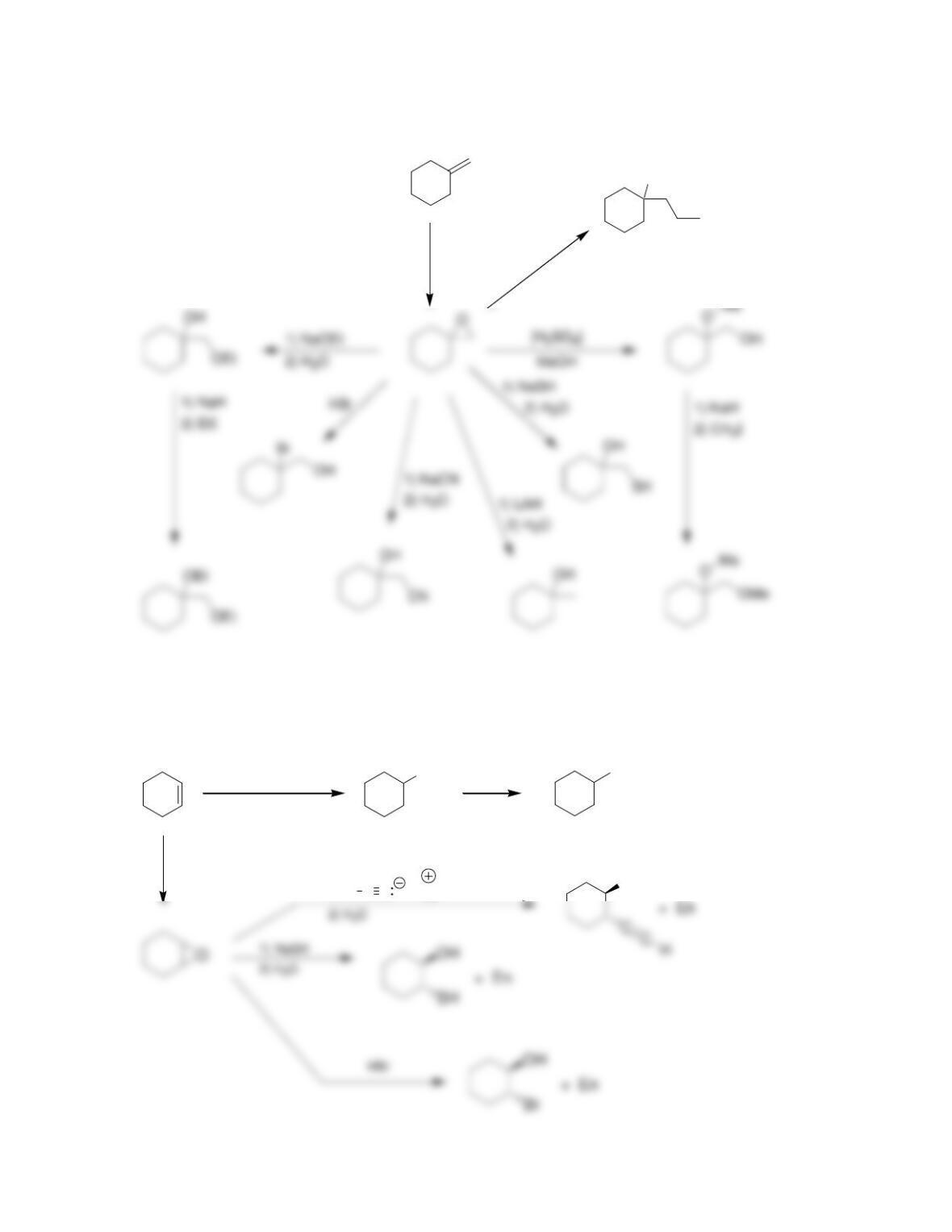
• Epoxides will undergo ring-opening reactions in: 1) conditions involving a
strong nucleophile, or under 2) _____-catalyzed conditions. When a strong
nucleophile is used, the nucleophile attacks at the ______-substituted position.