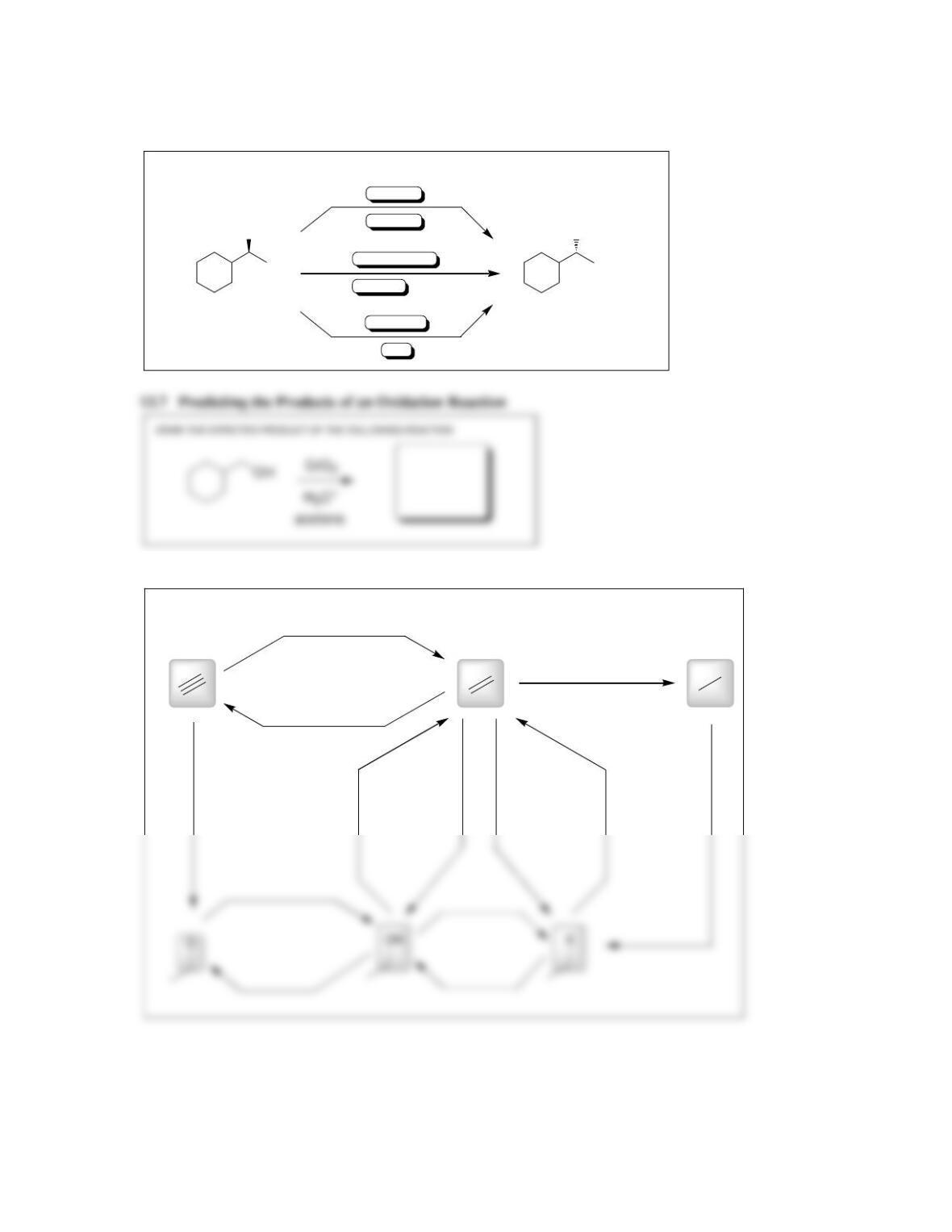
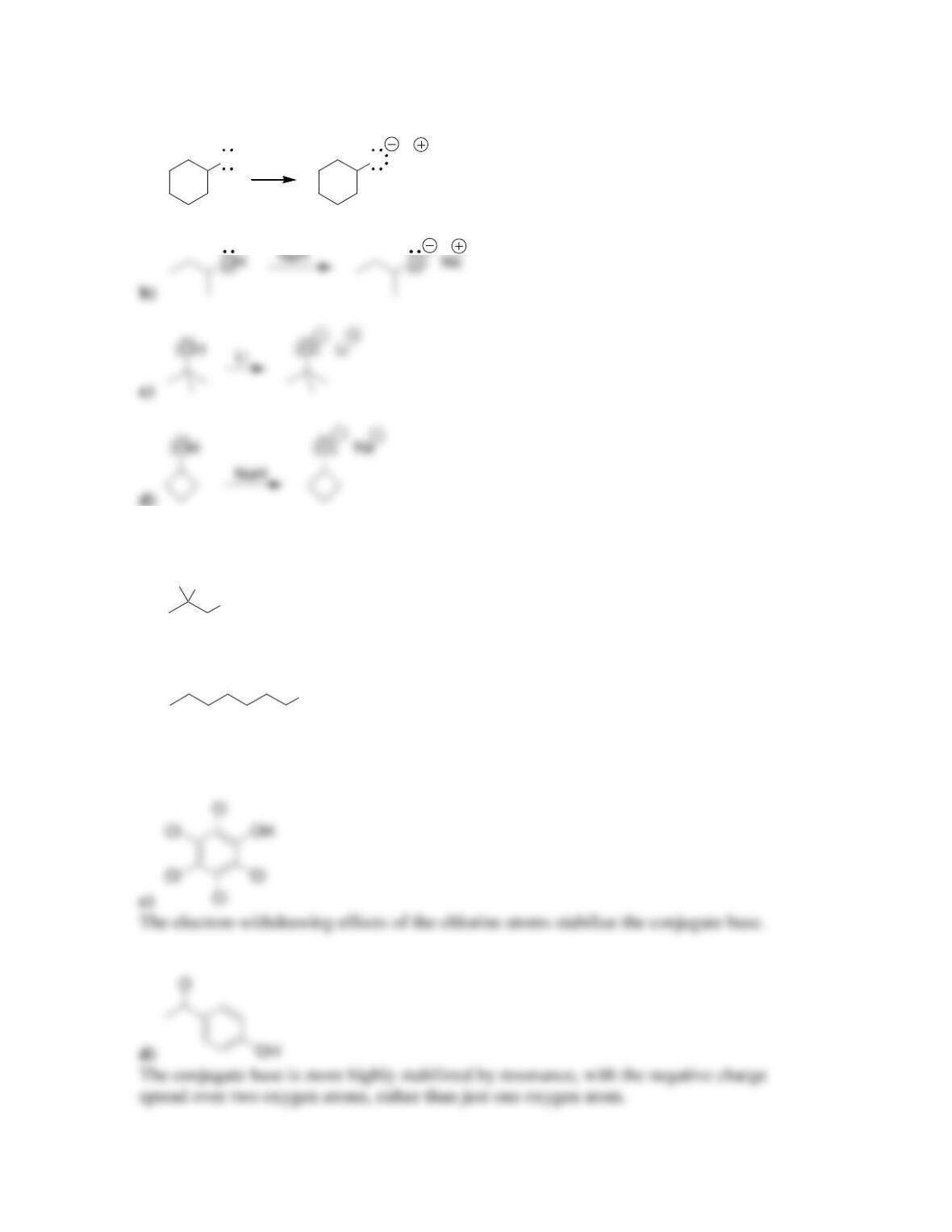
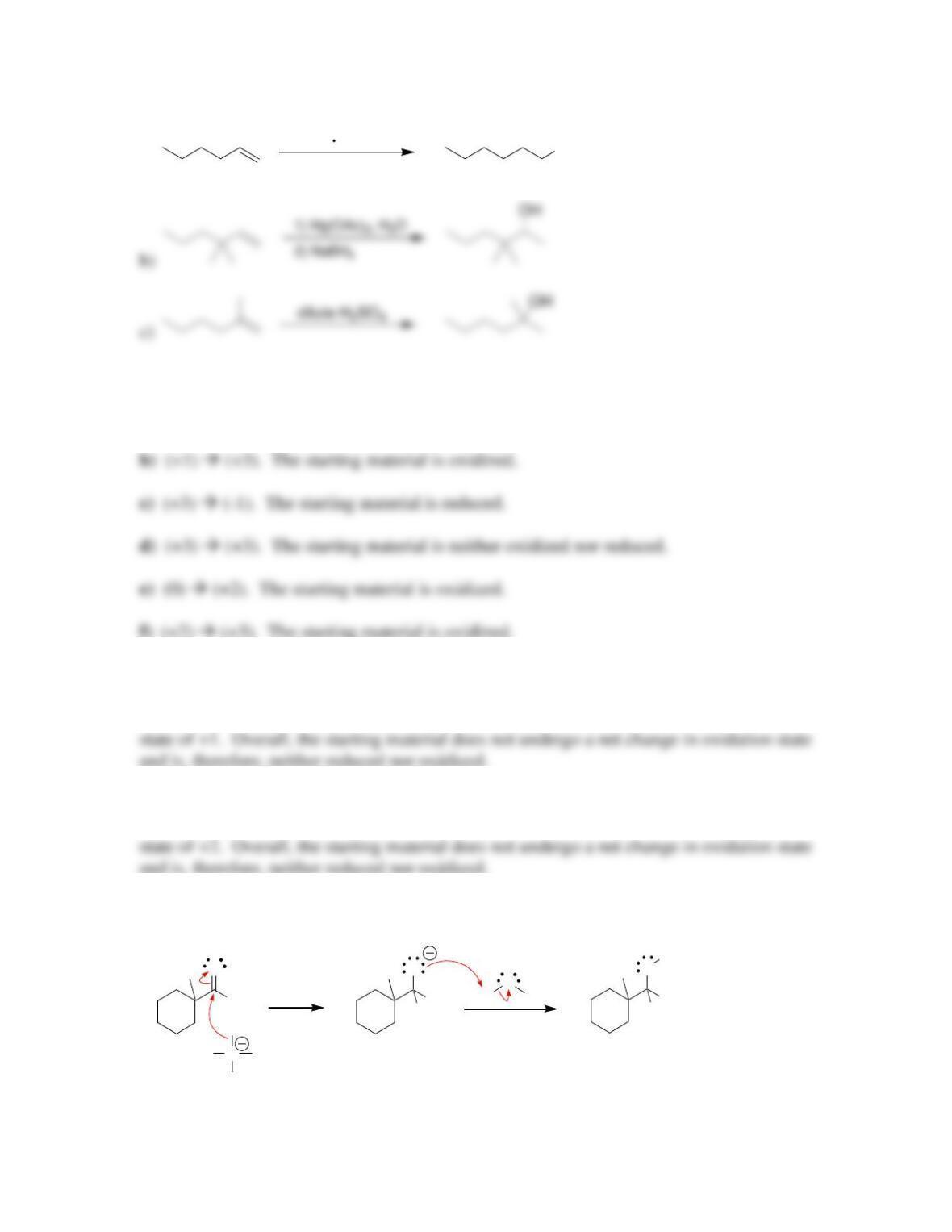
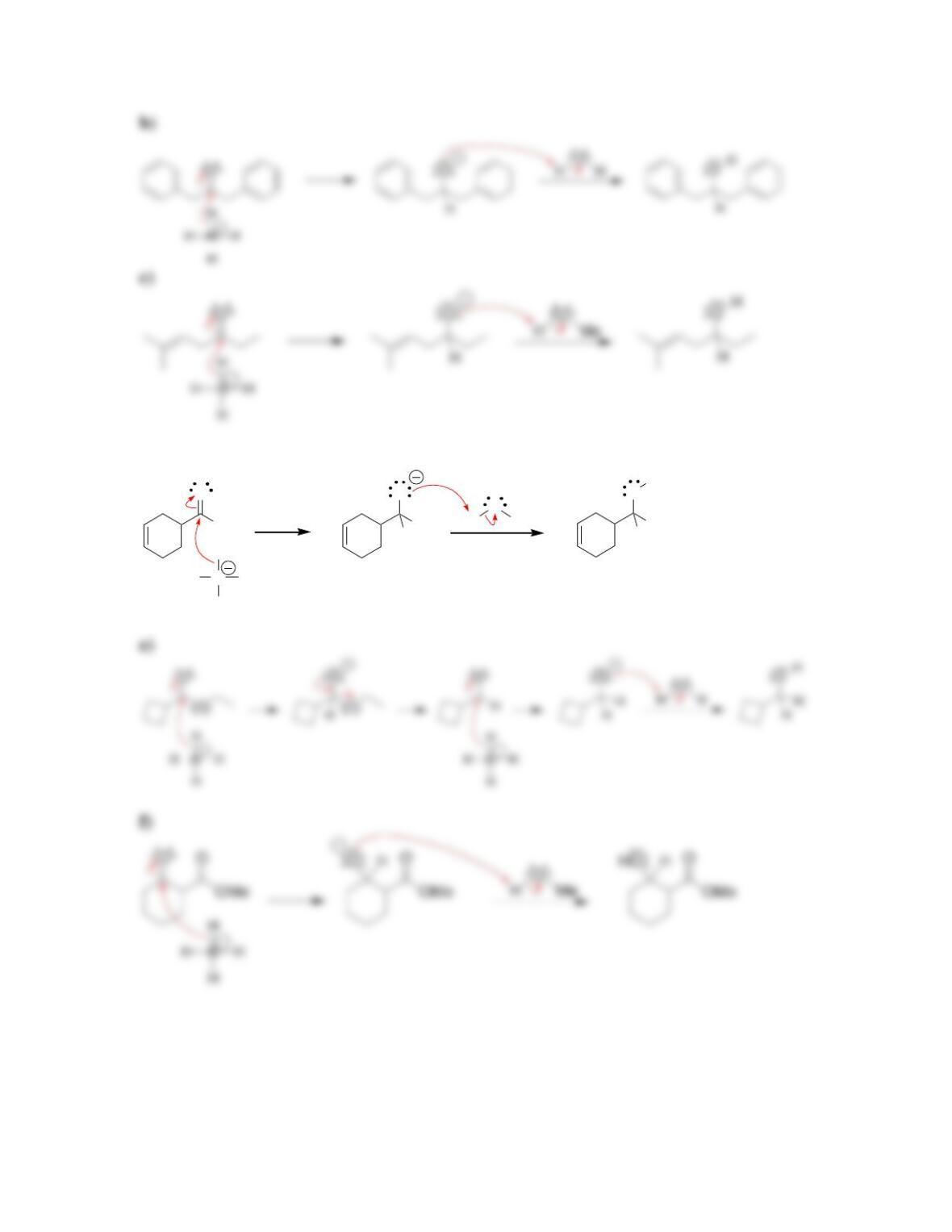
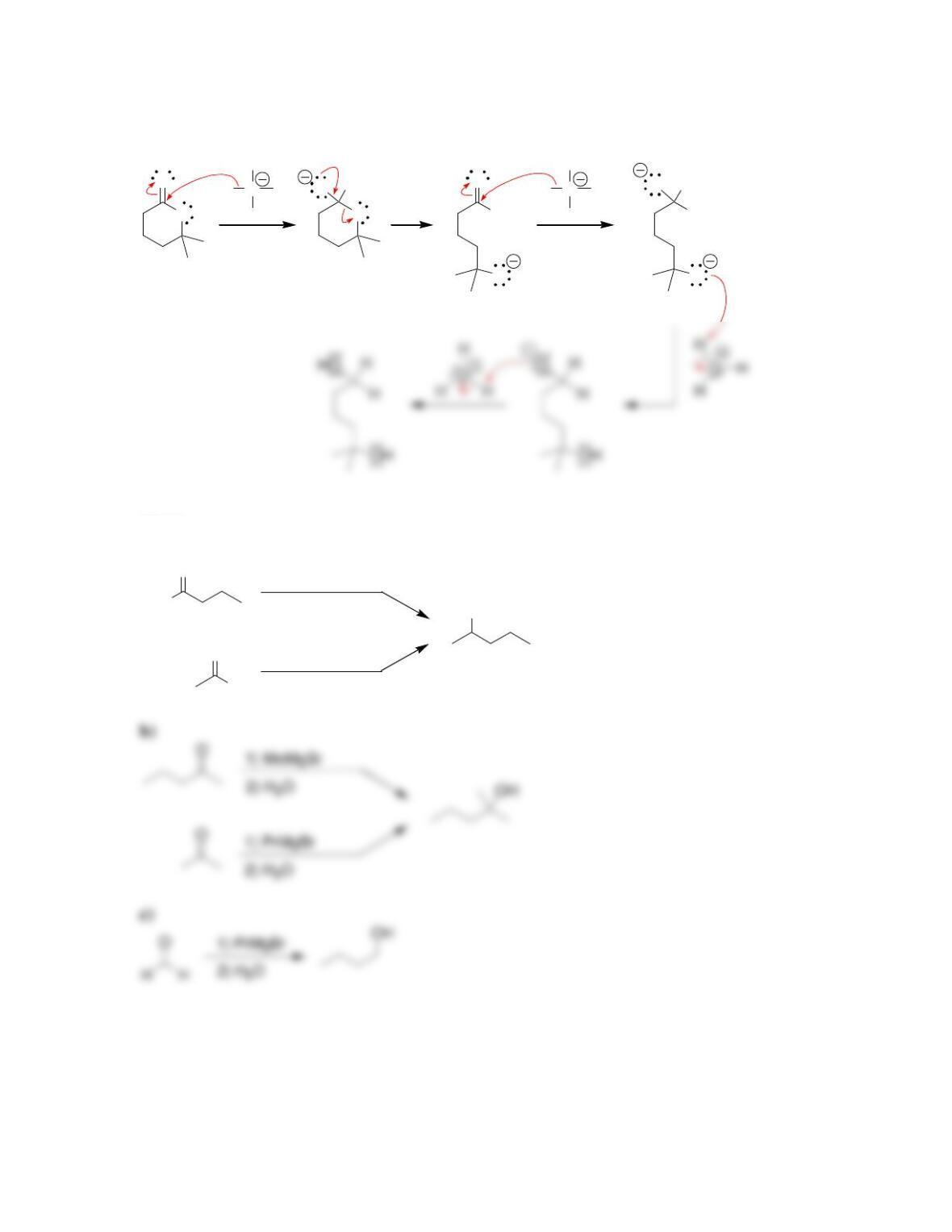
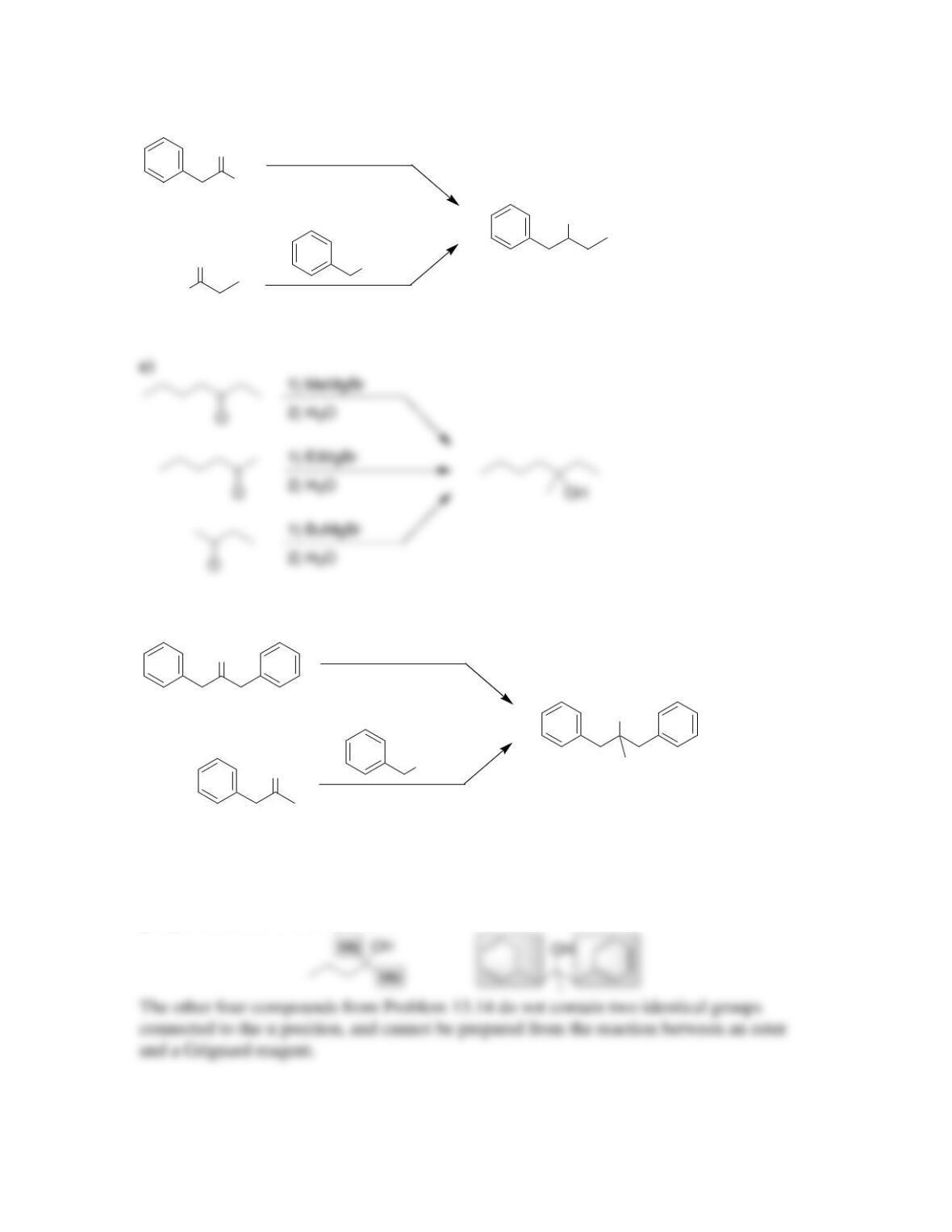
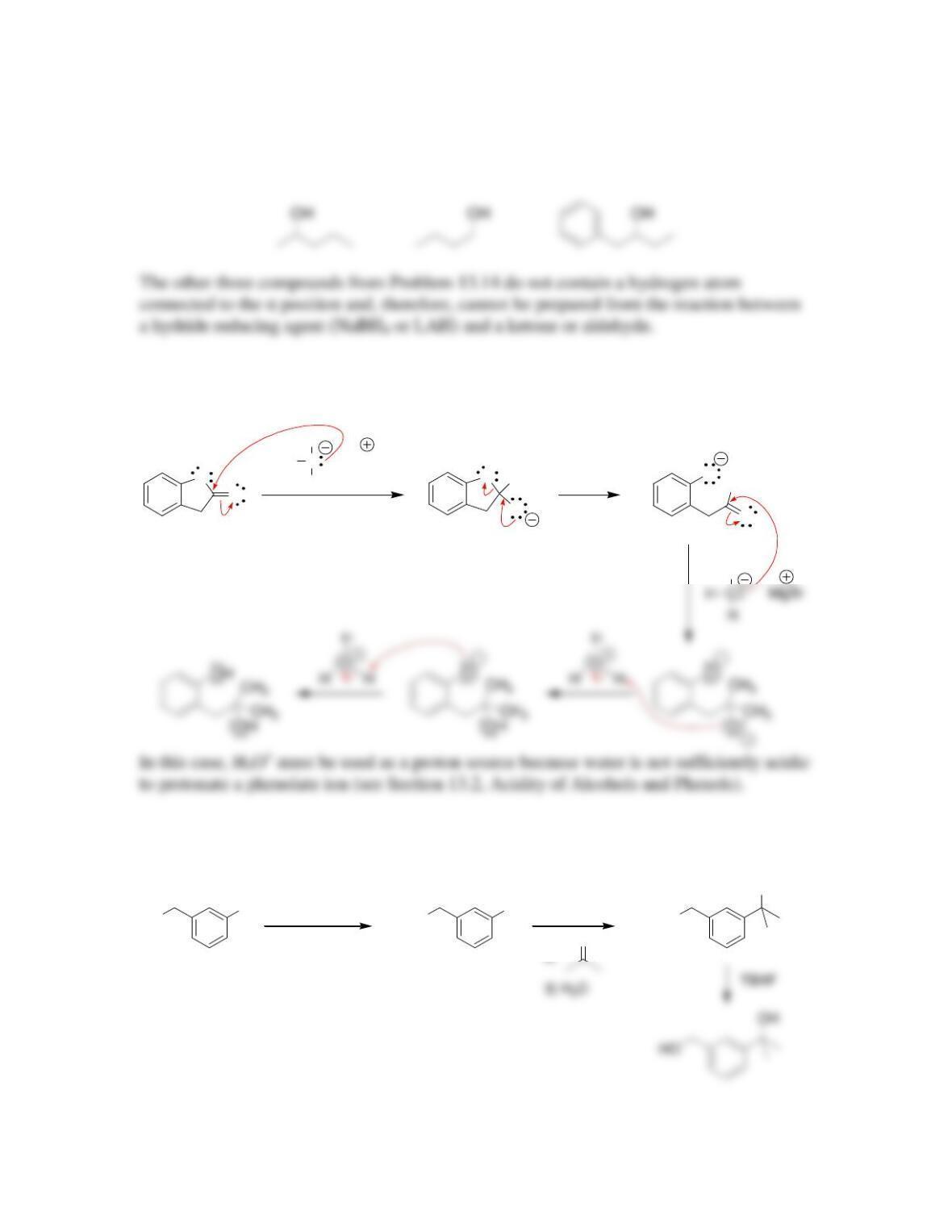
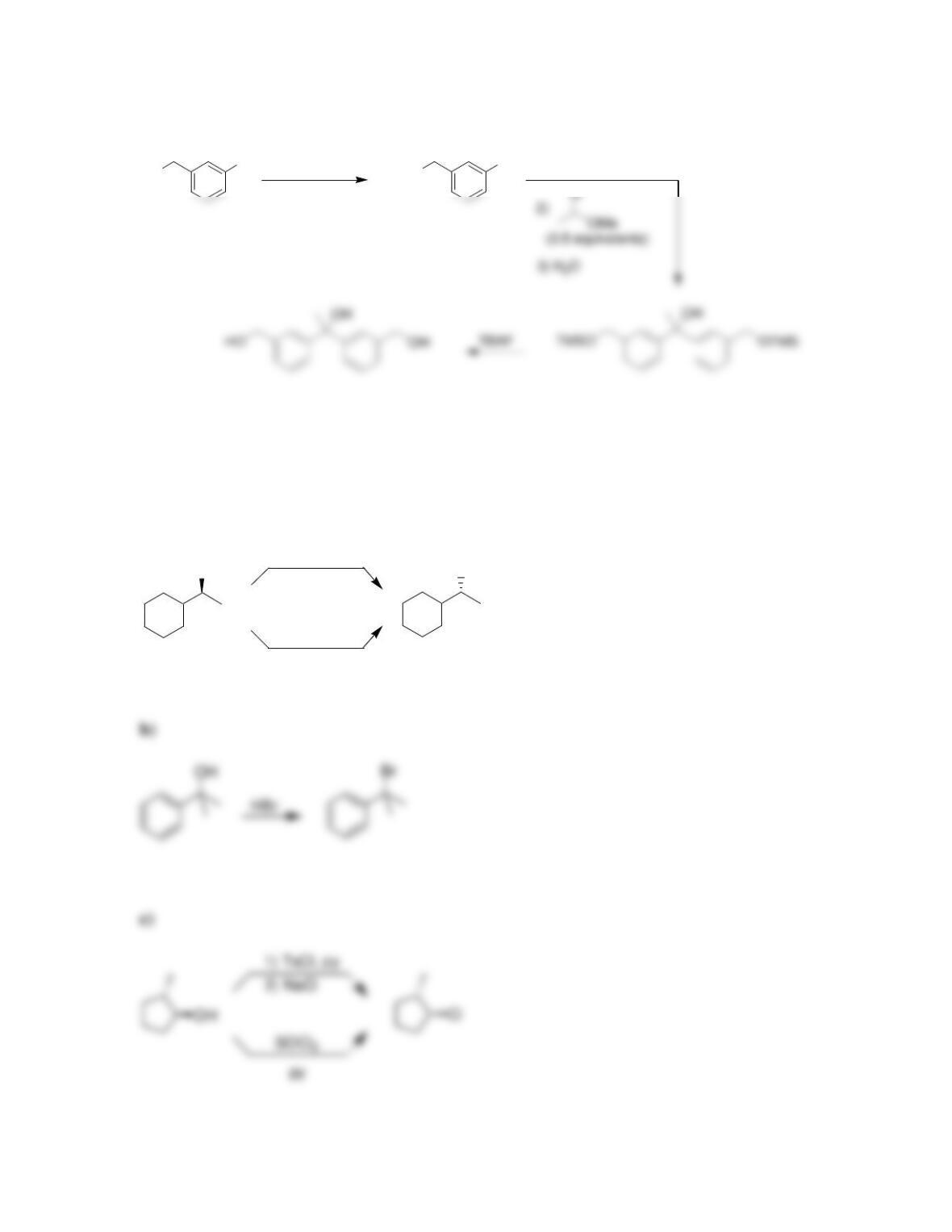
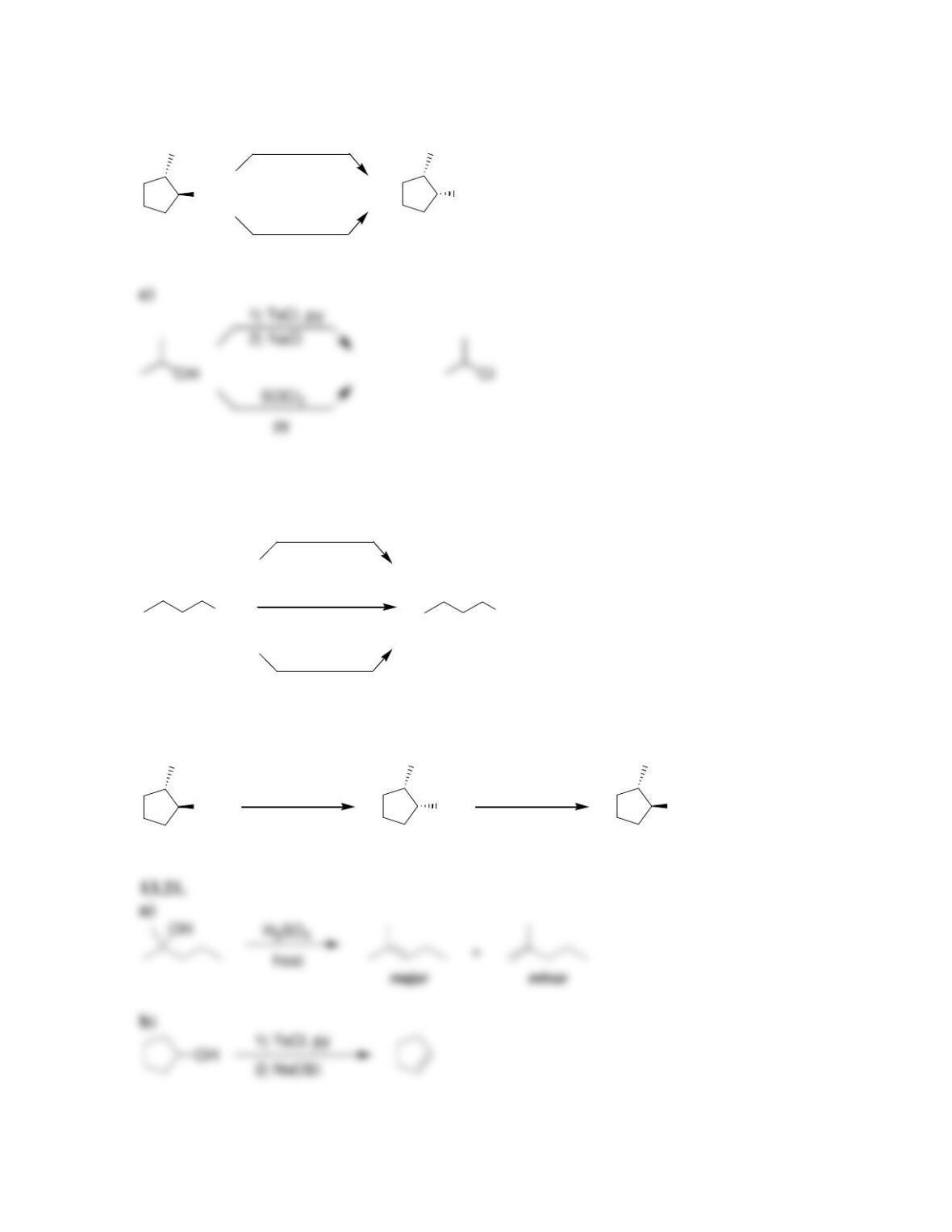
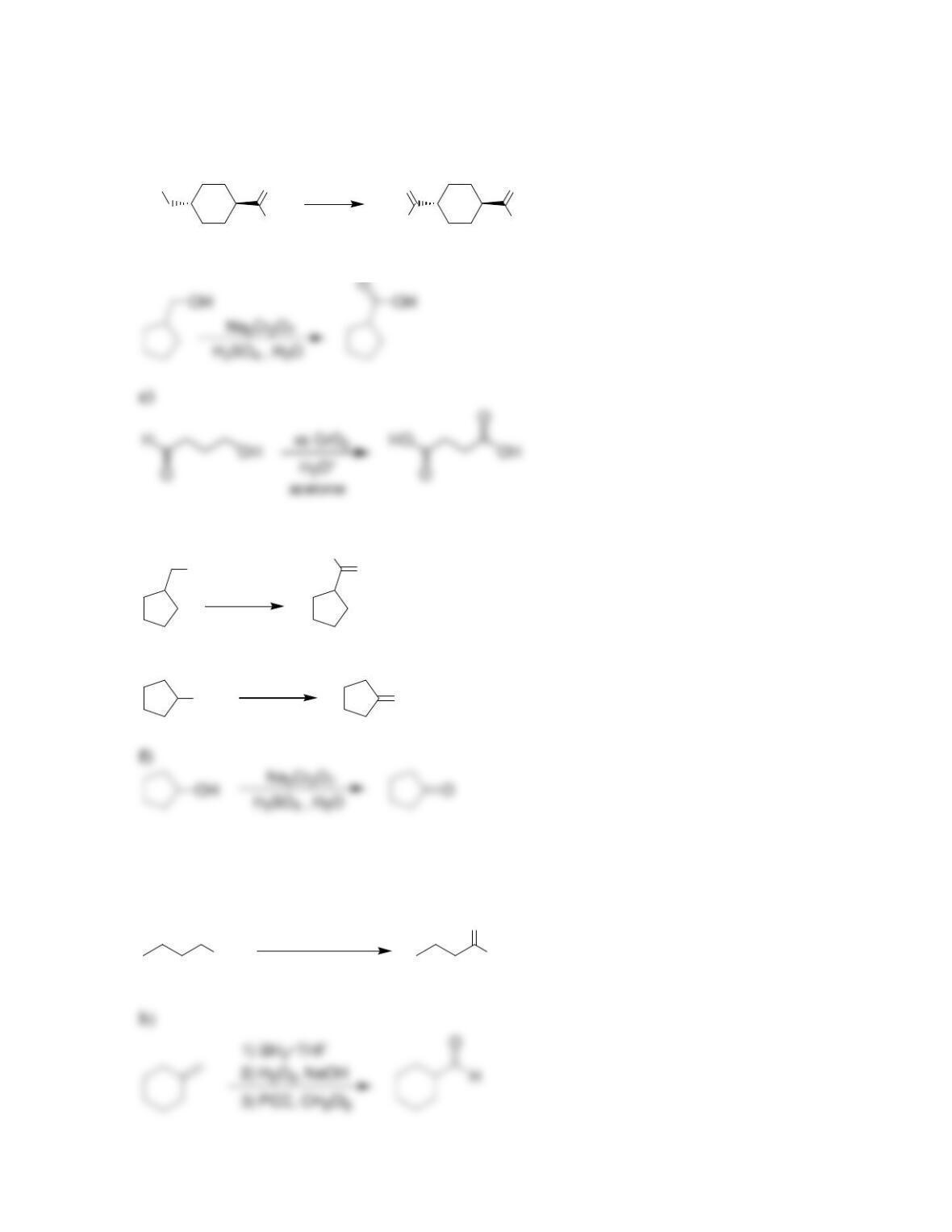
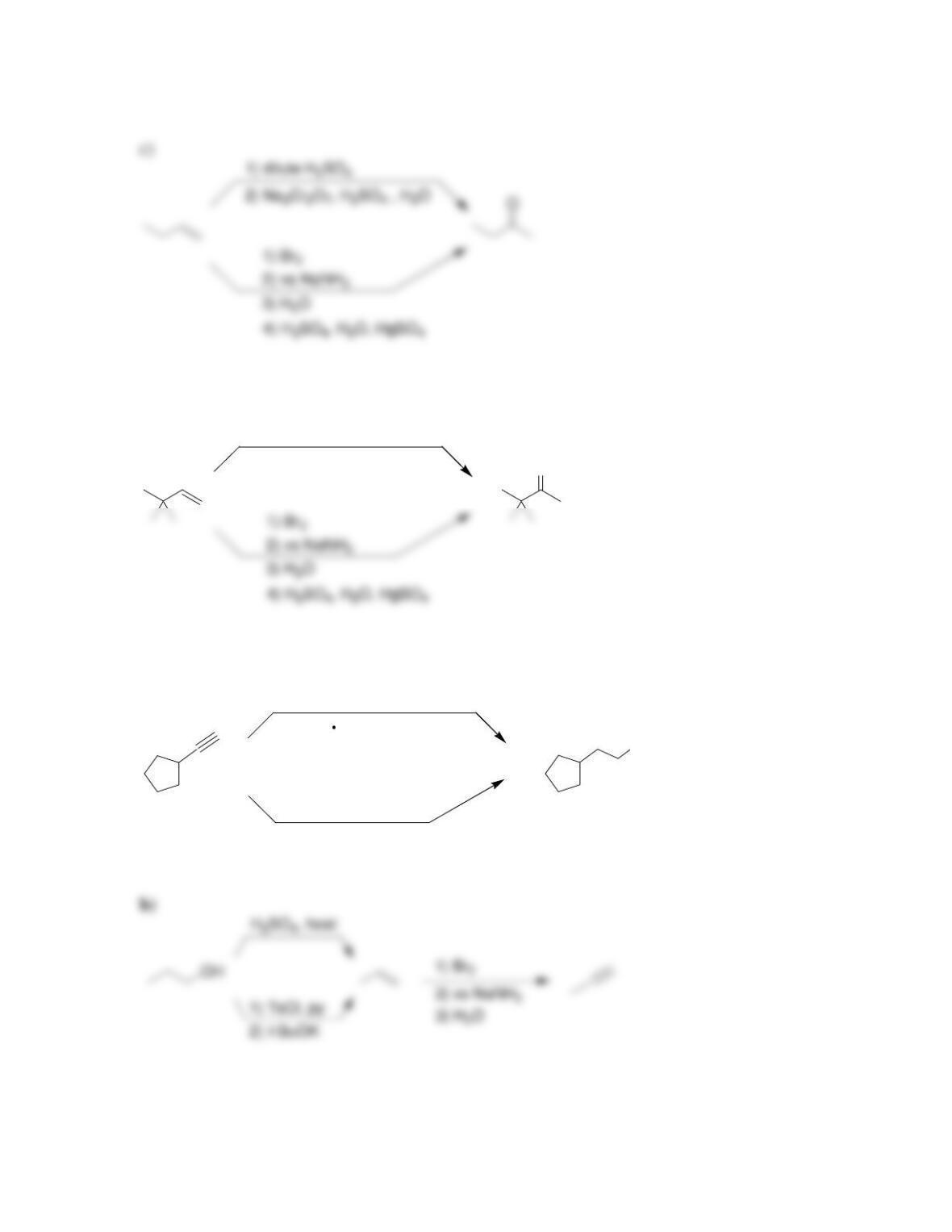
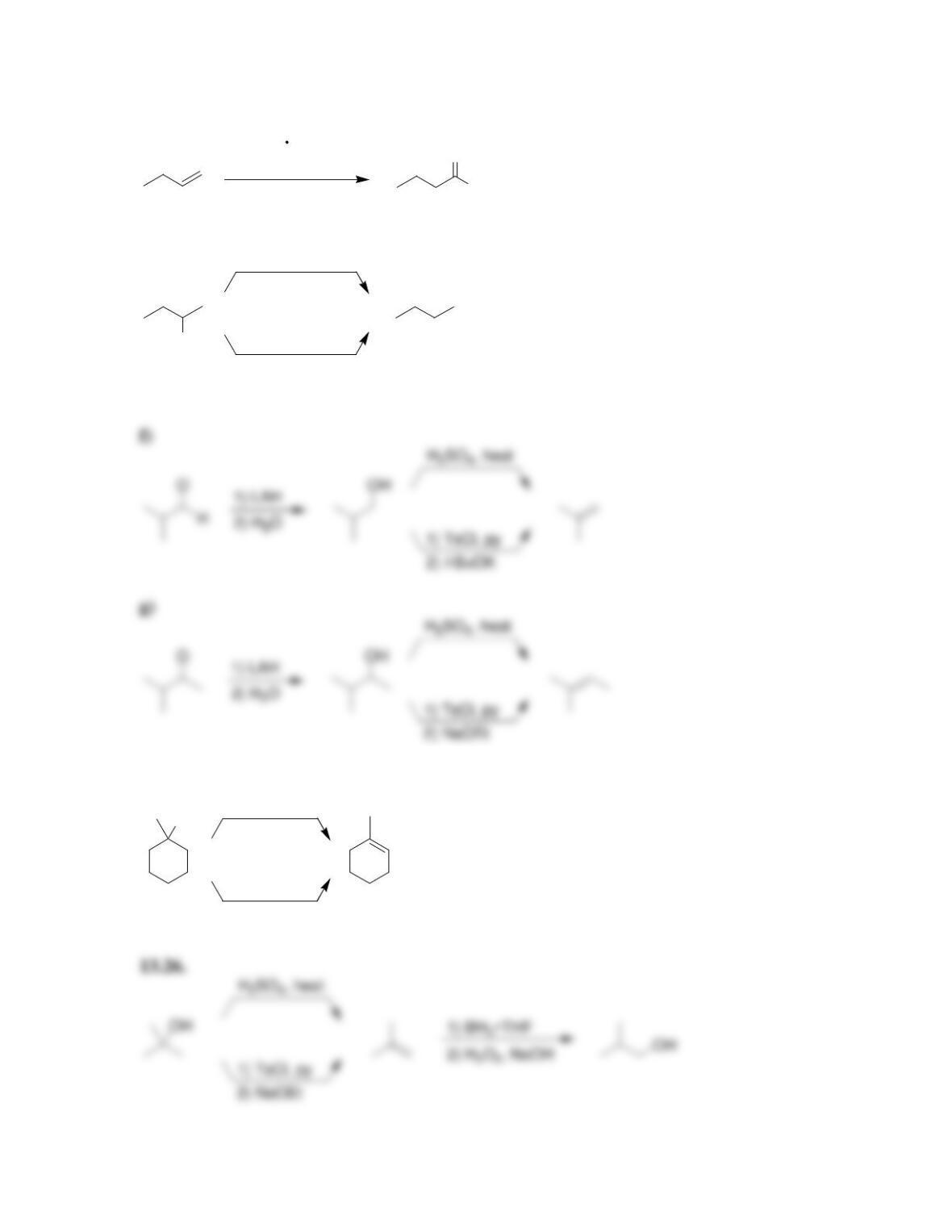
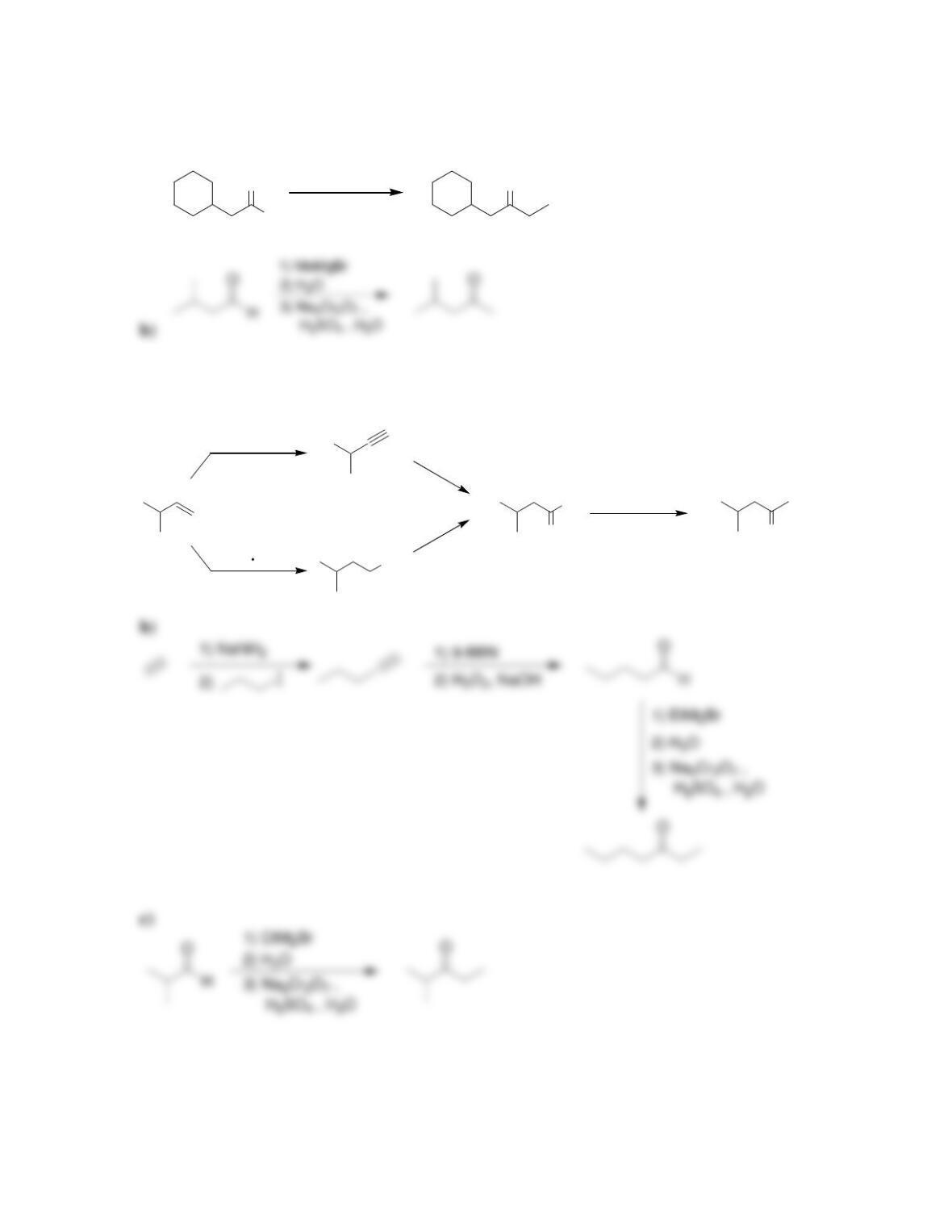
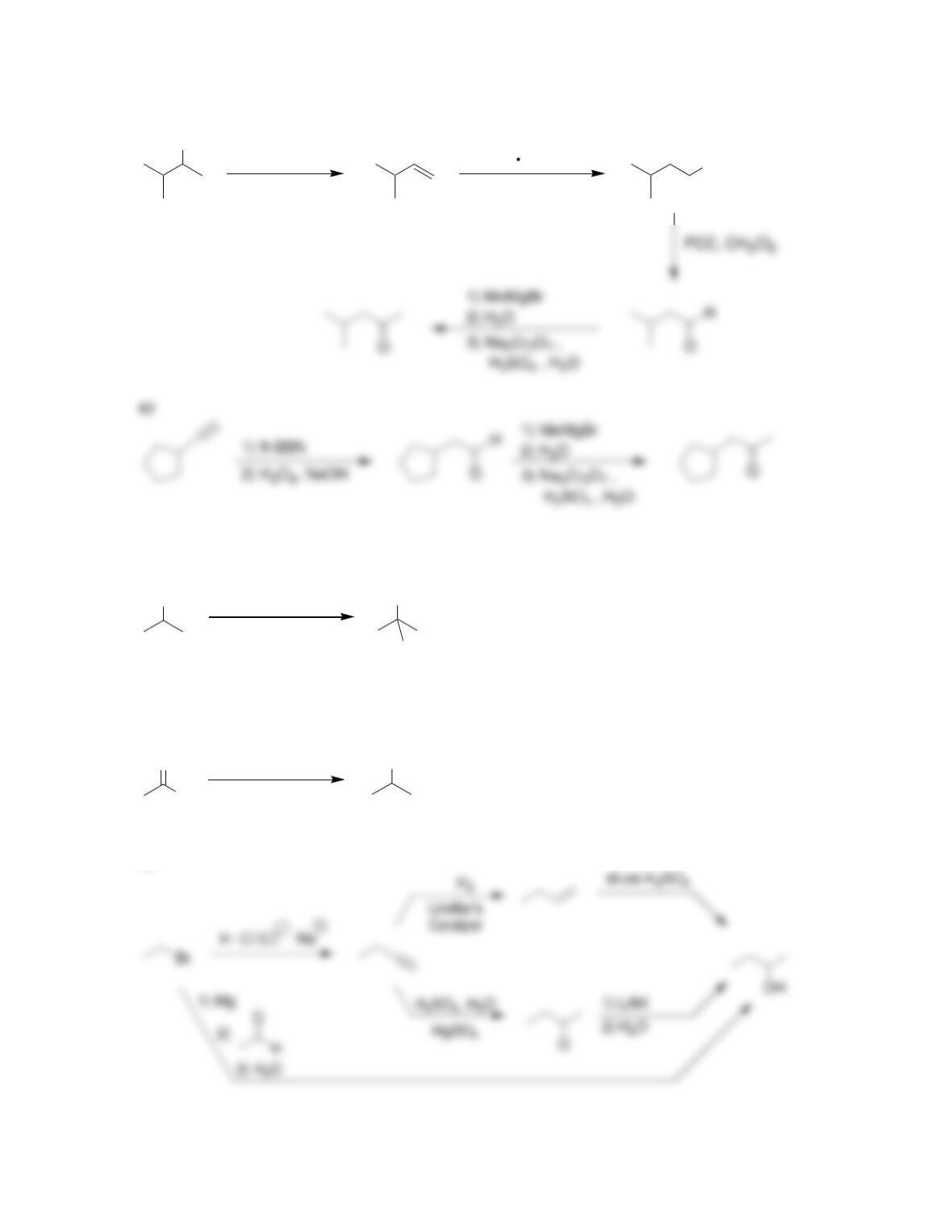
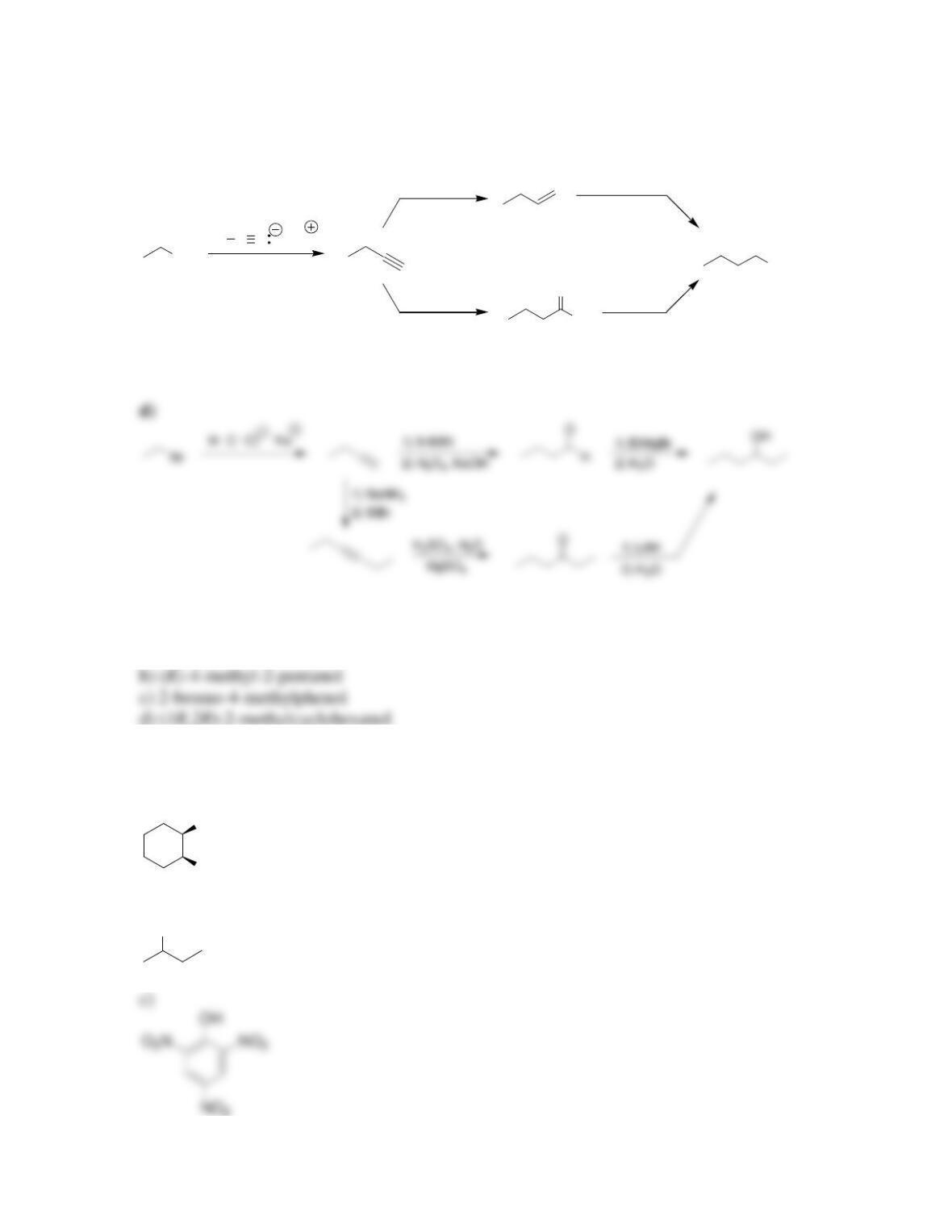
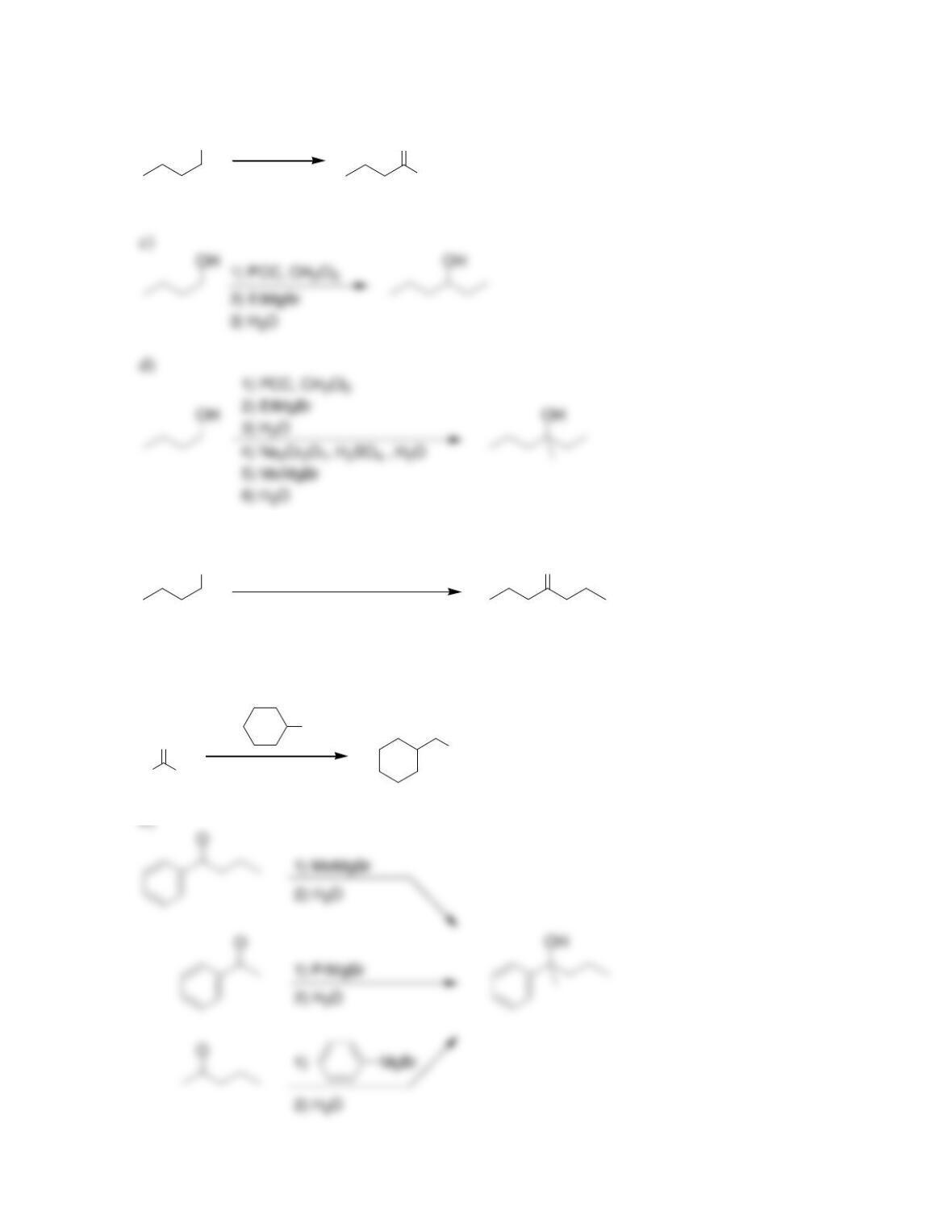
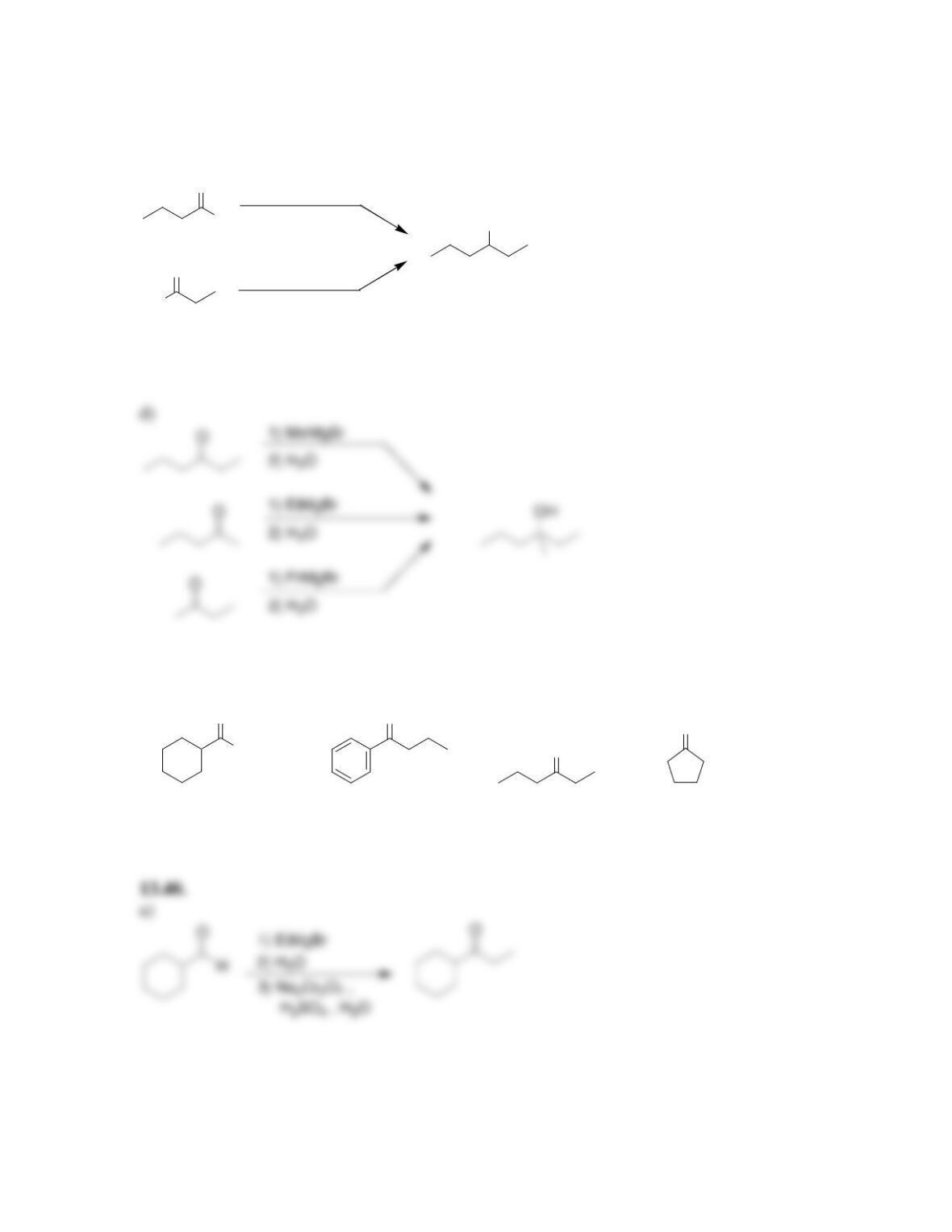
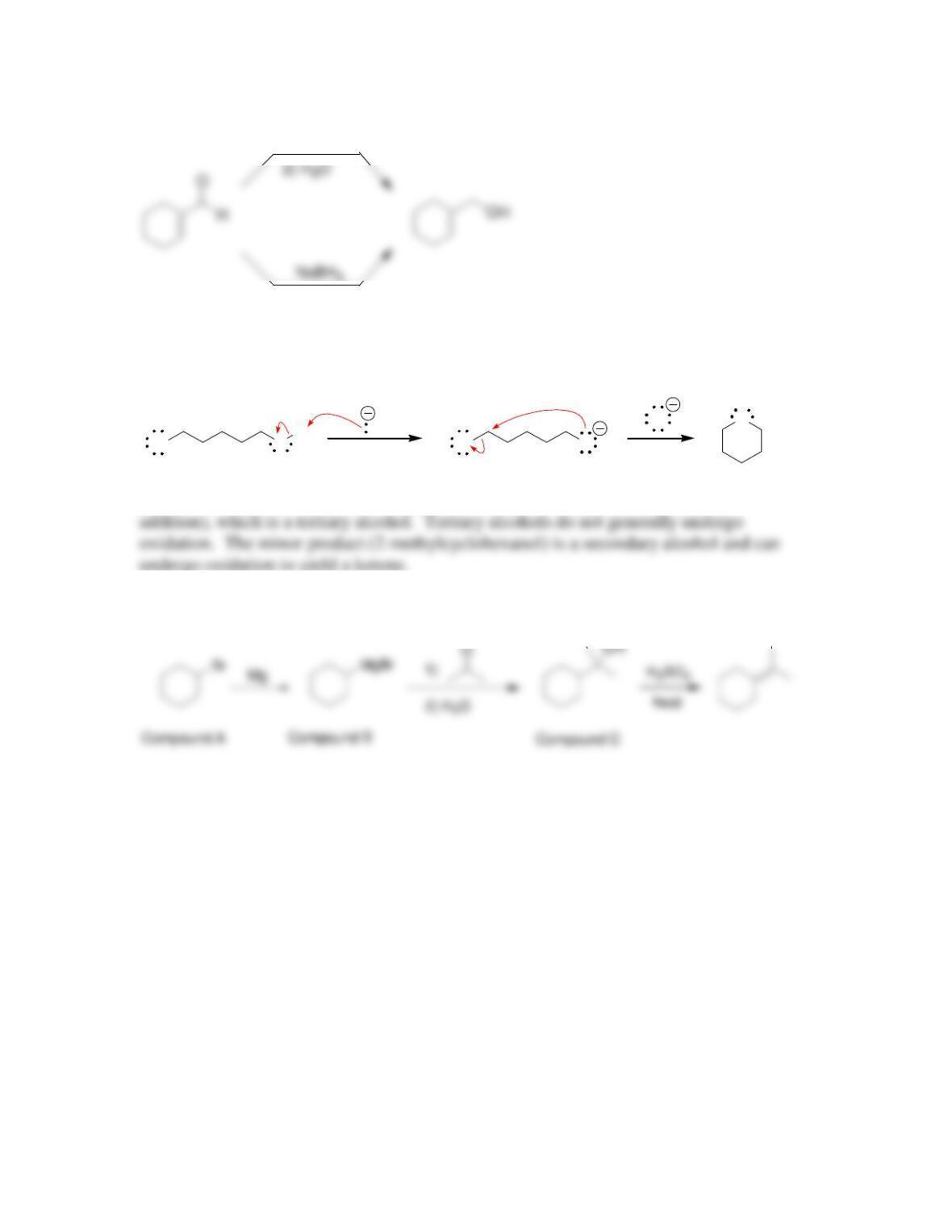
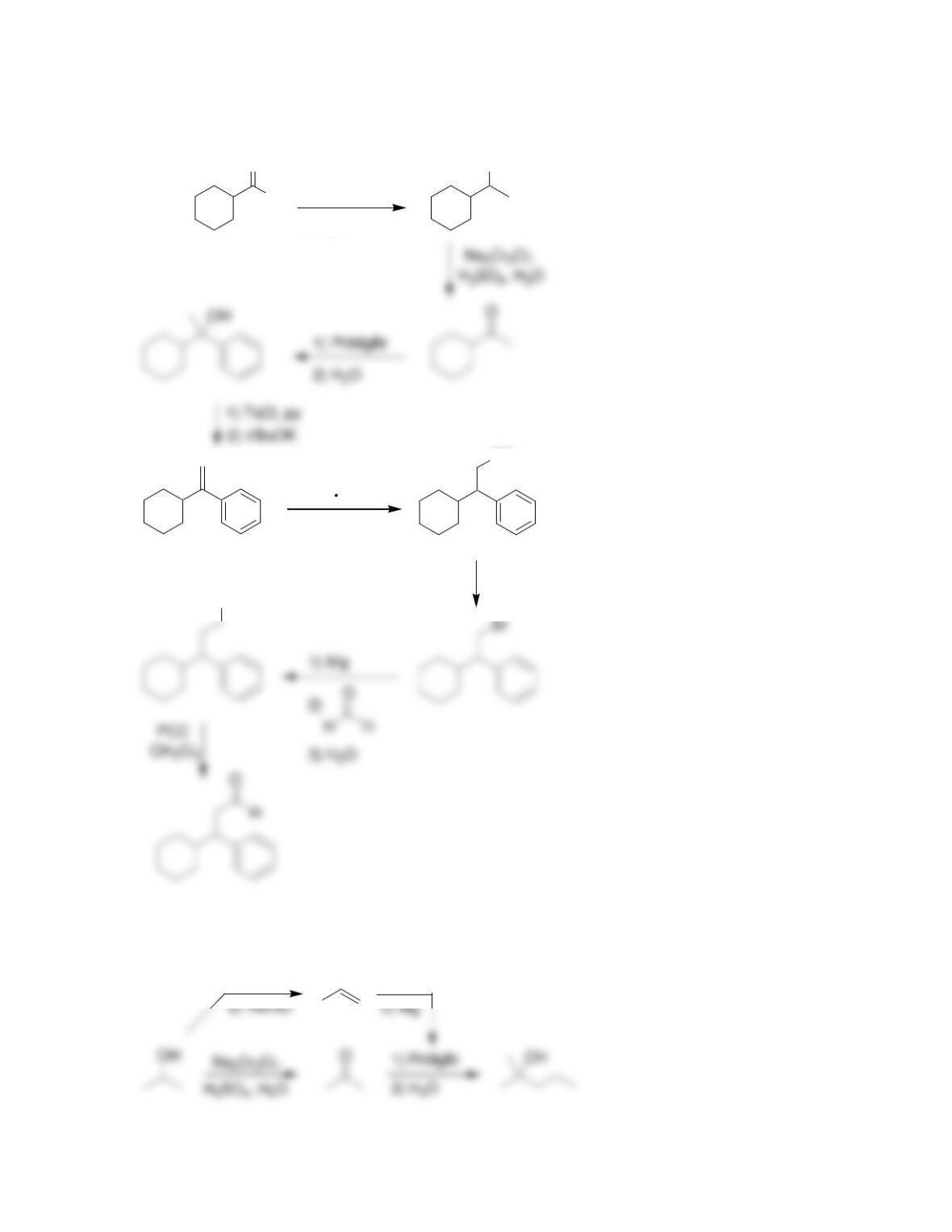
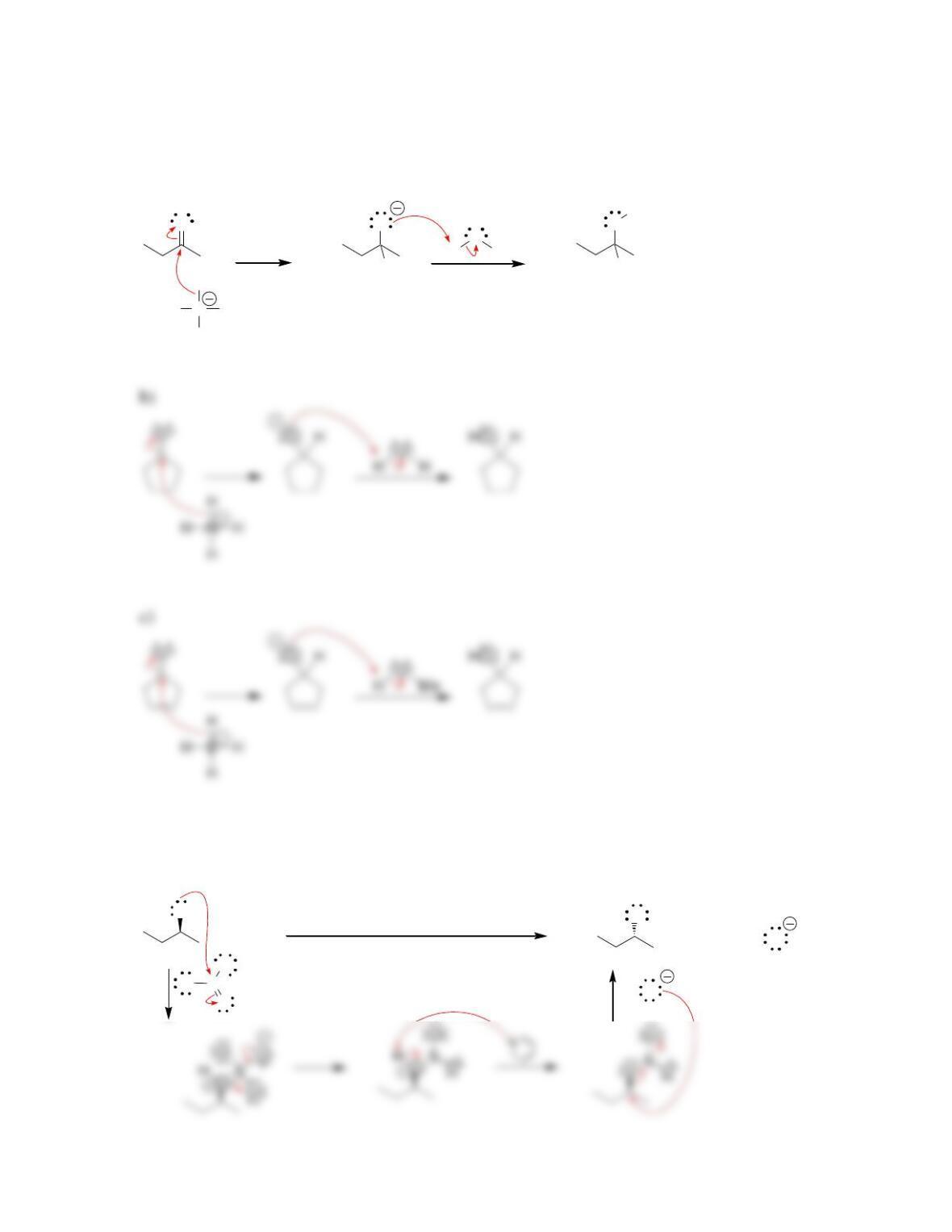
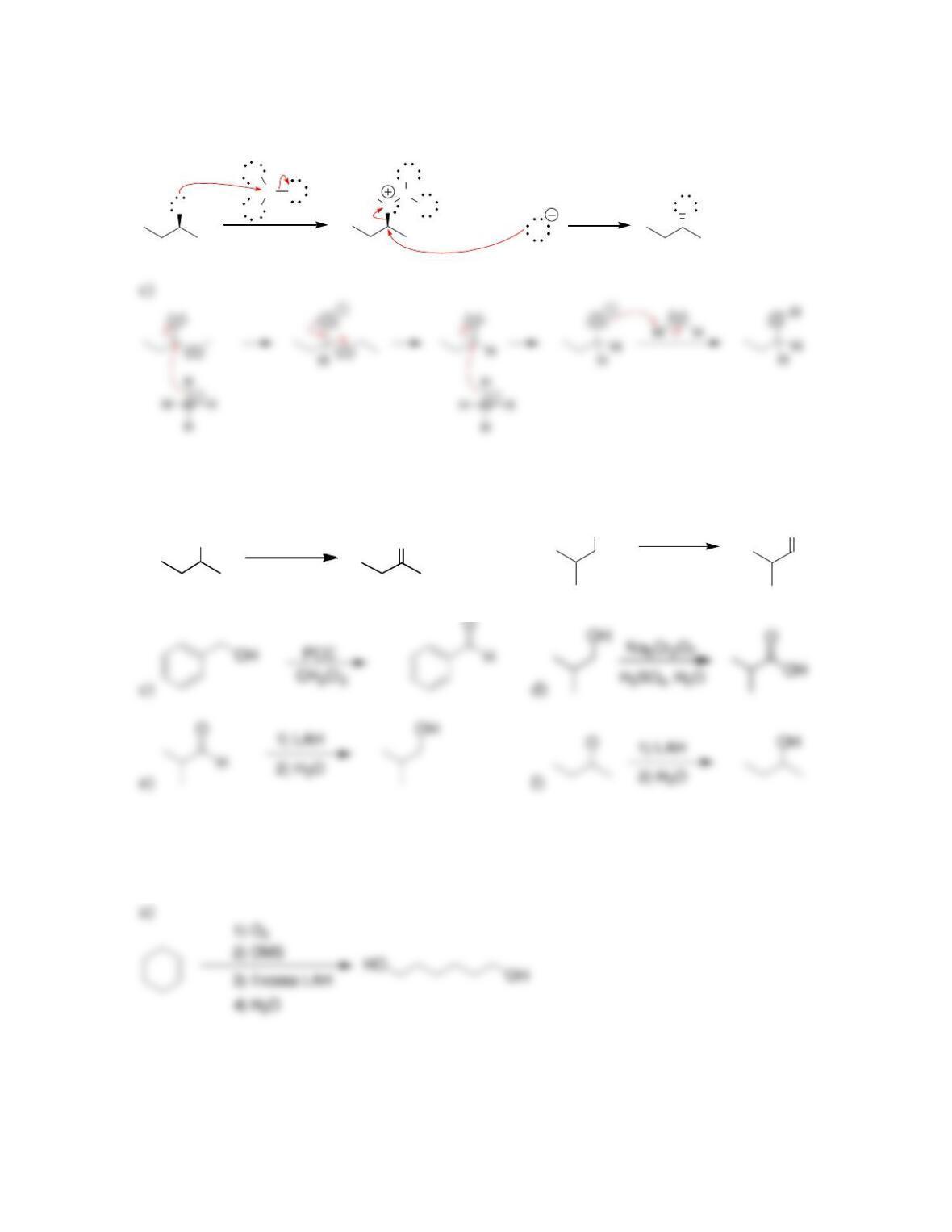
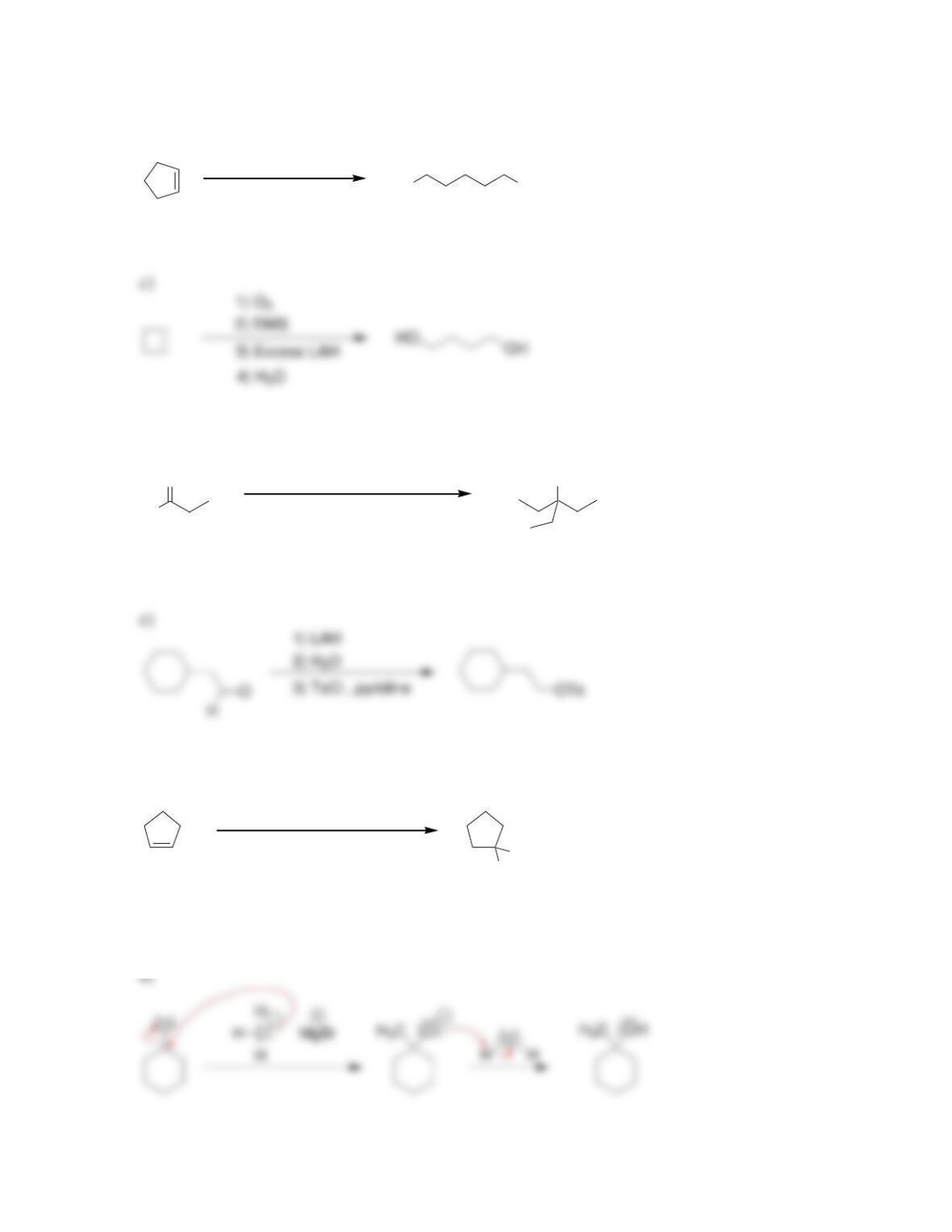
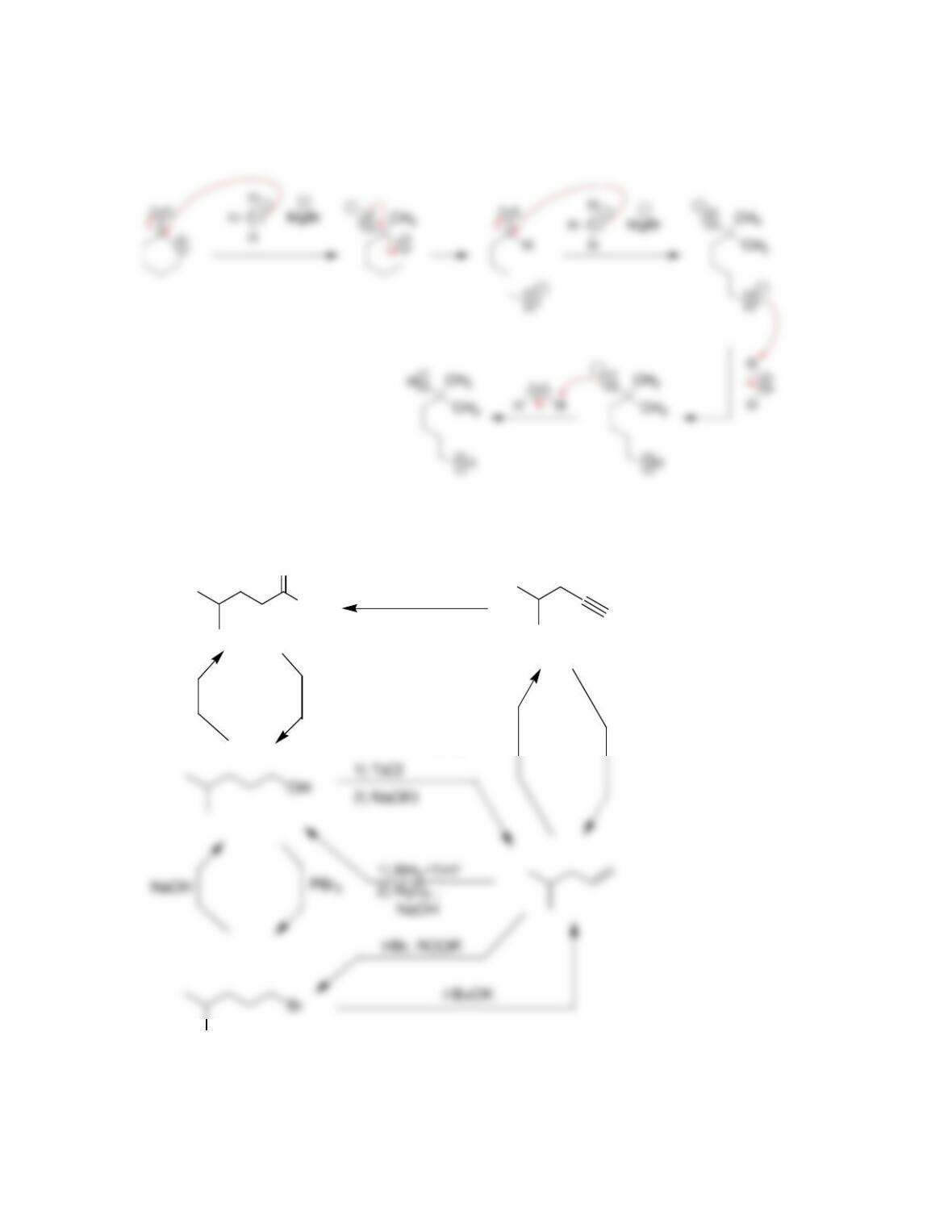
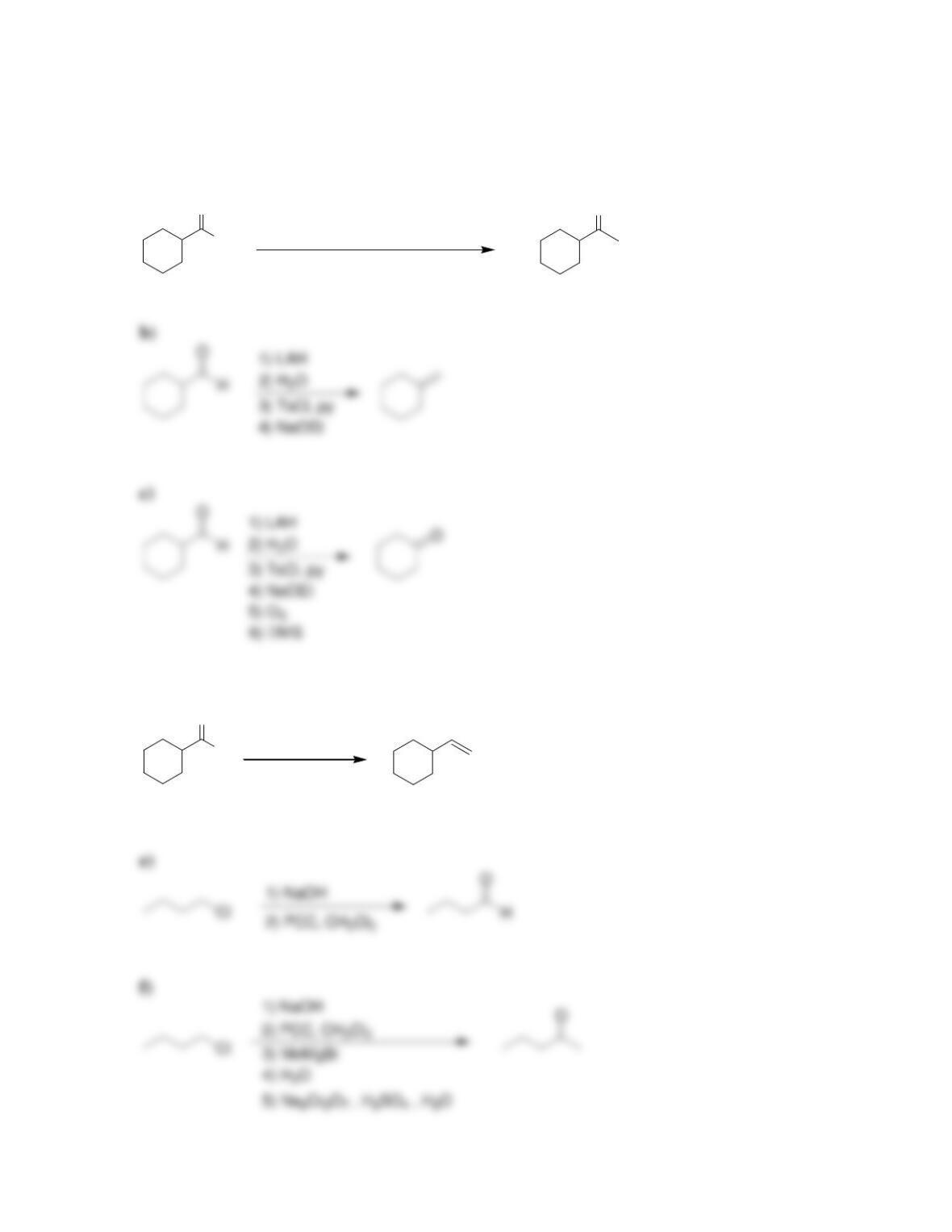
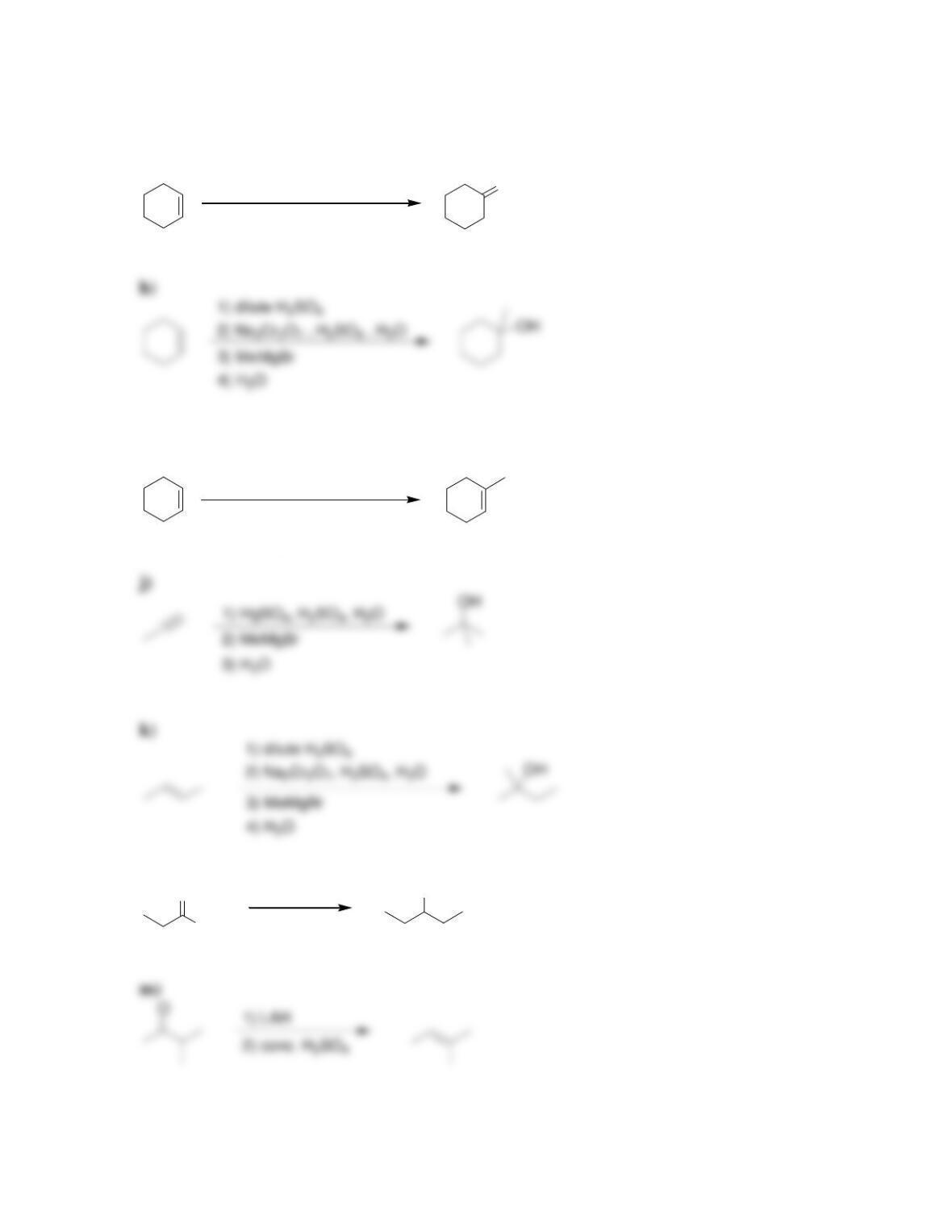
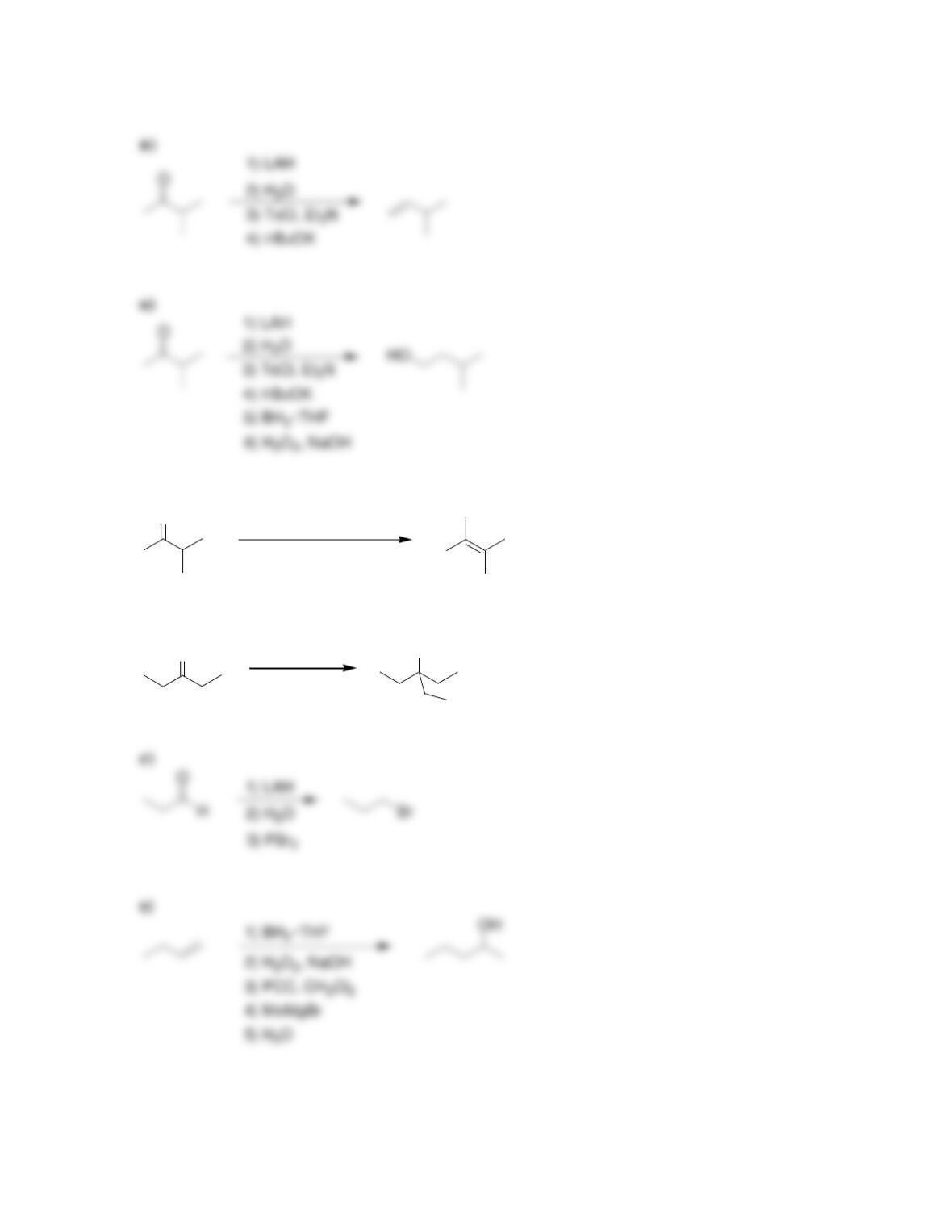
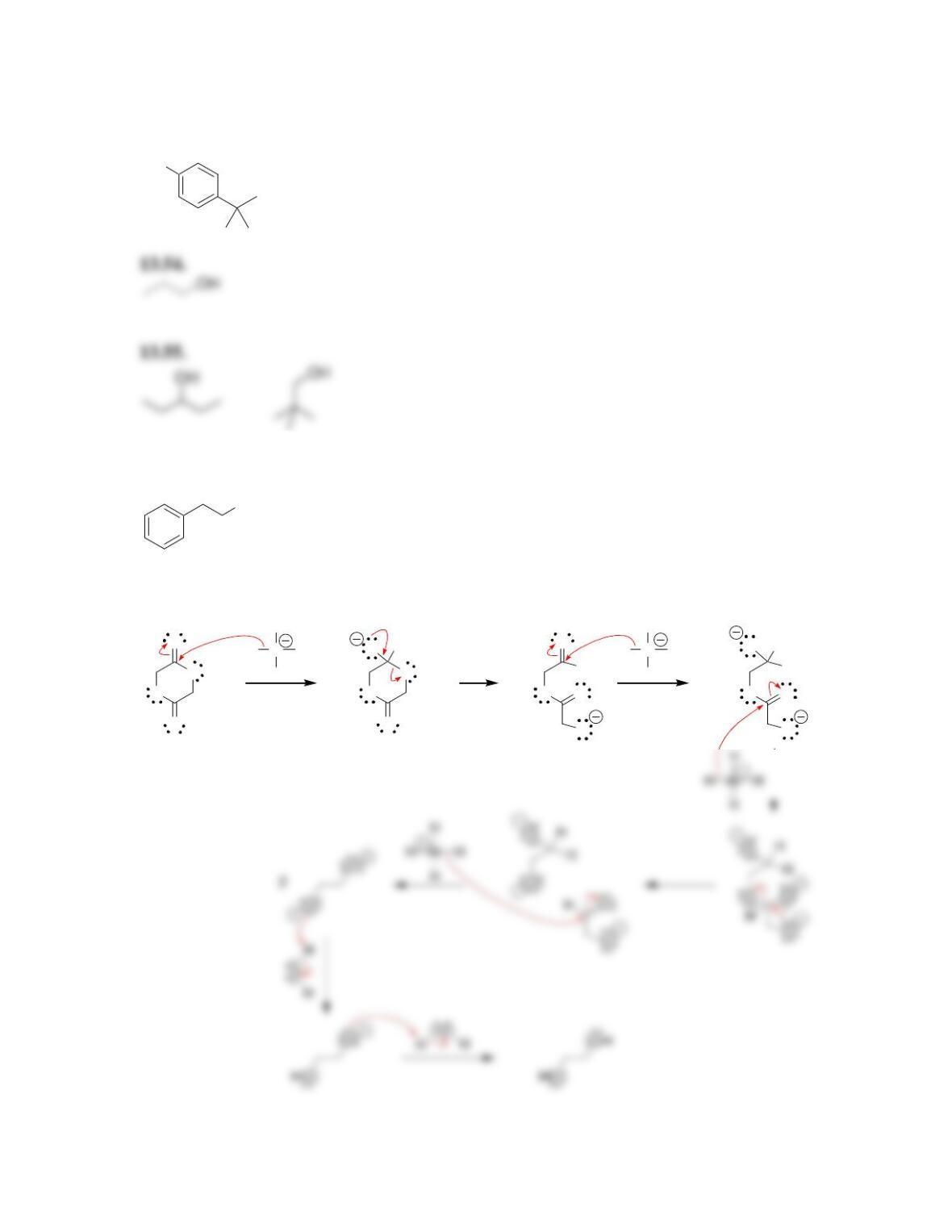
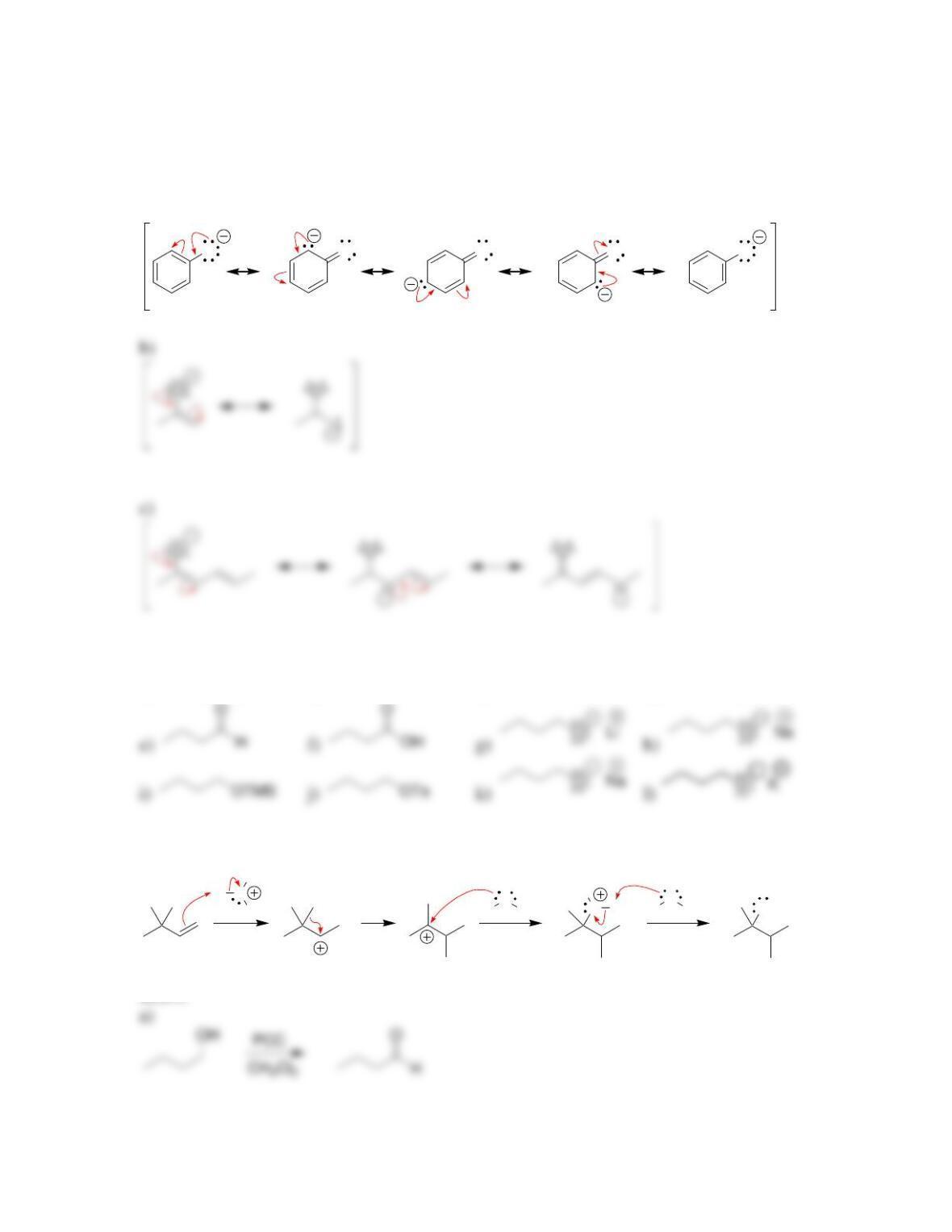Chapter 13
Alcohols
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 13. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• When naming an alcohol, the parent is the longest chain containing the
__________ group.
• The conjugate base of an alcohol is called an ____________ ion.
• Several factors determine the relative acidity of alcohols, including ___________,
____________, and _______________________.
• The conjugate base of phenol is called a ____________, or _____________ ion.
• Primary and secondary alcohols will undergo an S
N
___ process when treated with
either HX, SOCl
2
, PBr
3
, or when the hydroxyl group is converted into a tosylate
group followed by nucleophilic attack.
• Tertiary alcohols undergo E1 elimination when treated with __________.
• Primary alcohols undergo oxidation twice to give a _____________________.
• Secondary alcohols are oxidized only once to give a ___________