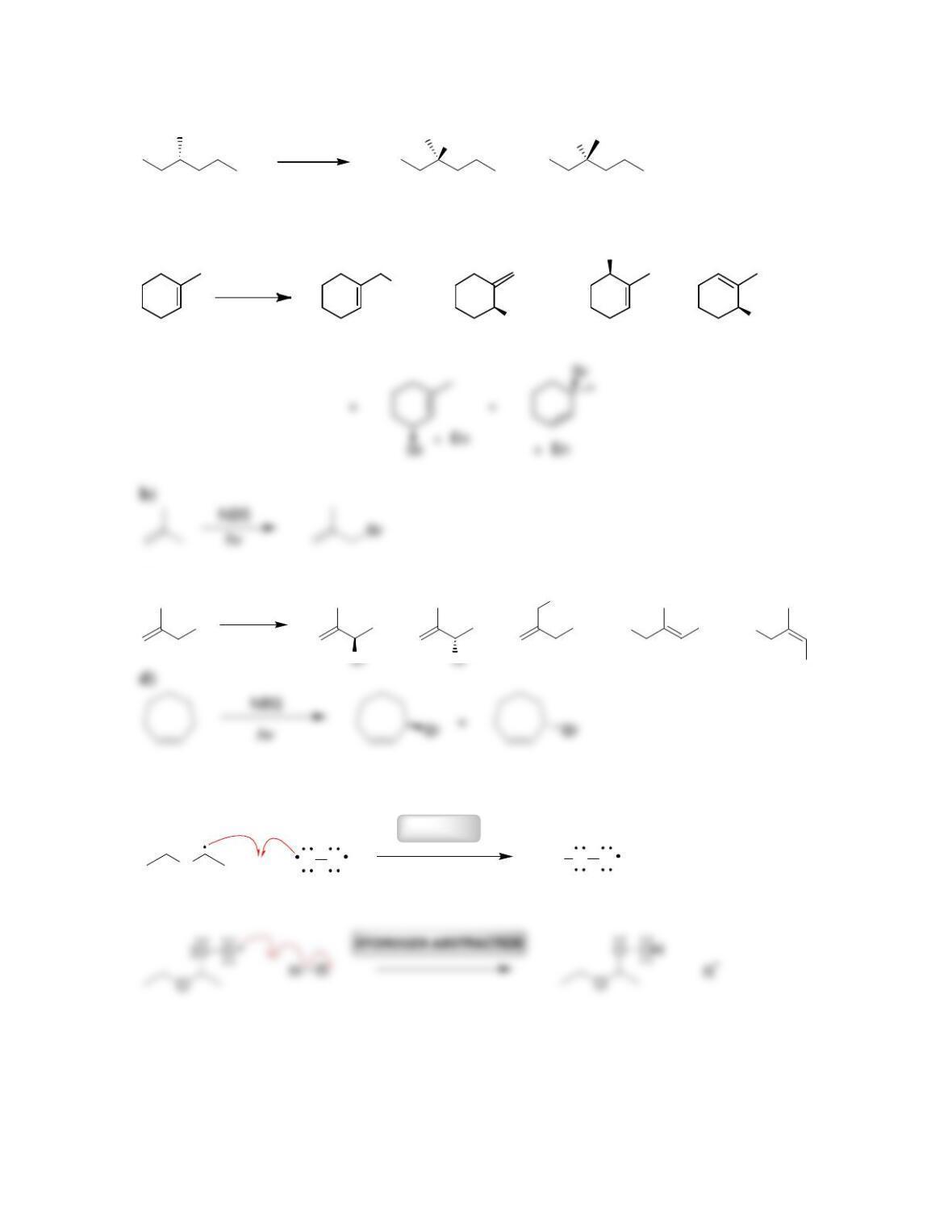
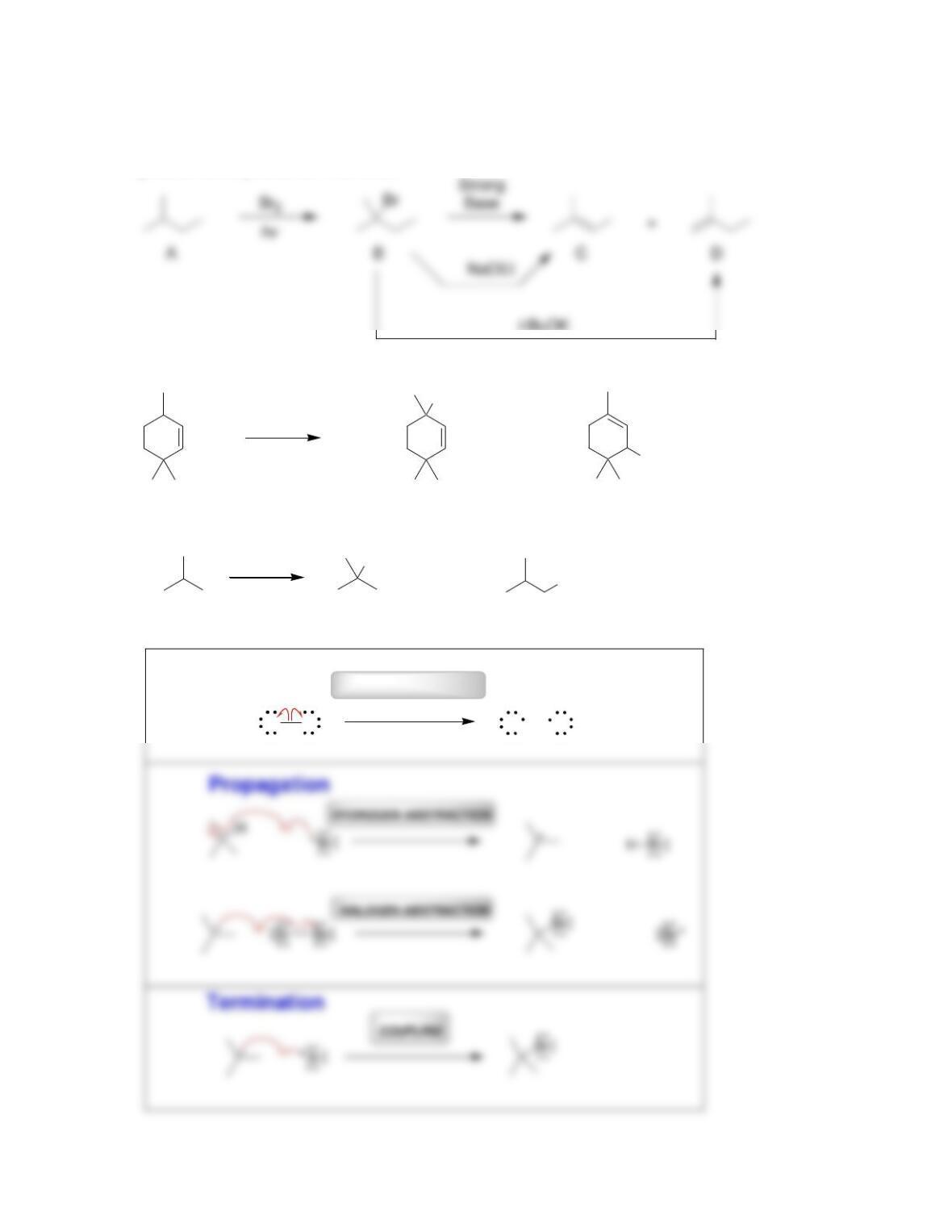
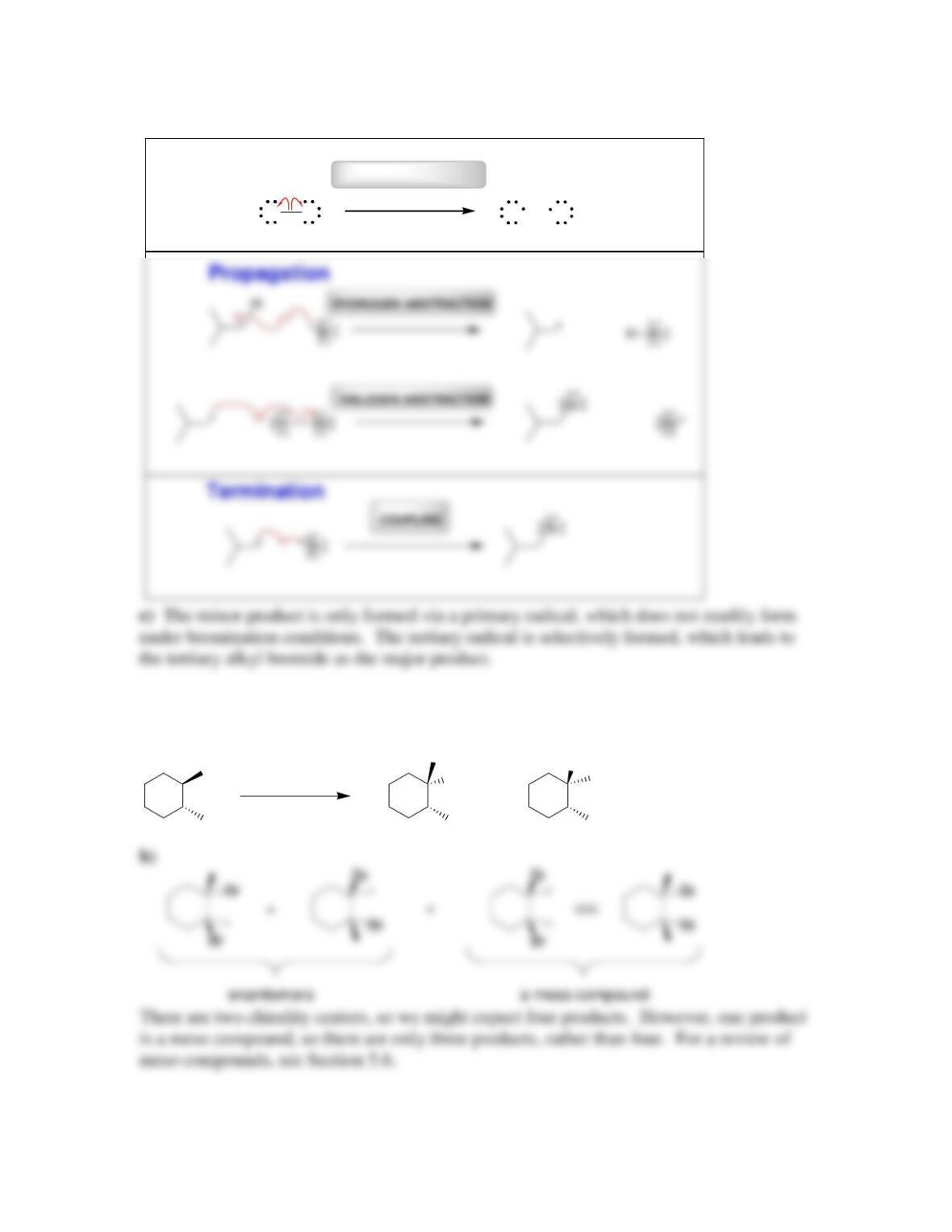
Chapter 11
Radical Reactions
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 11. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
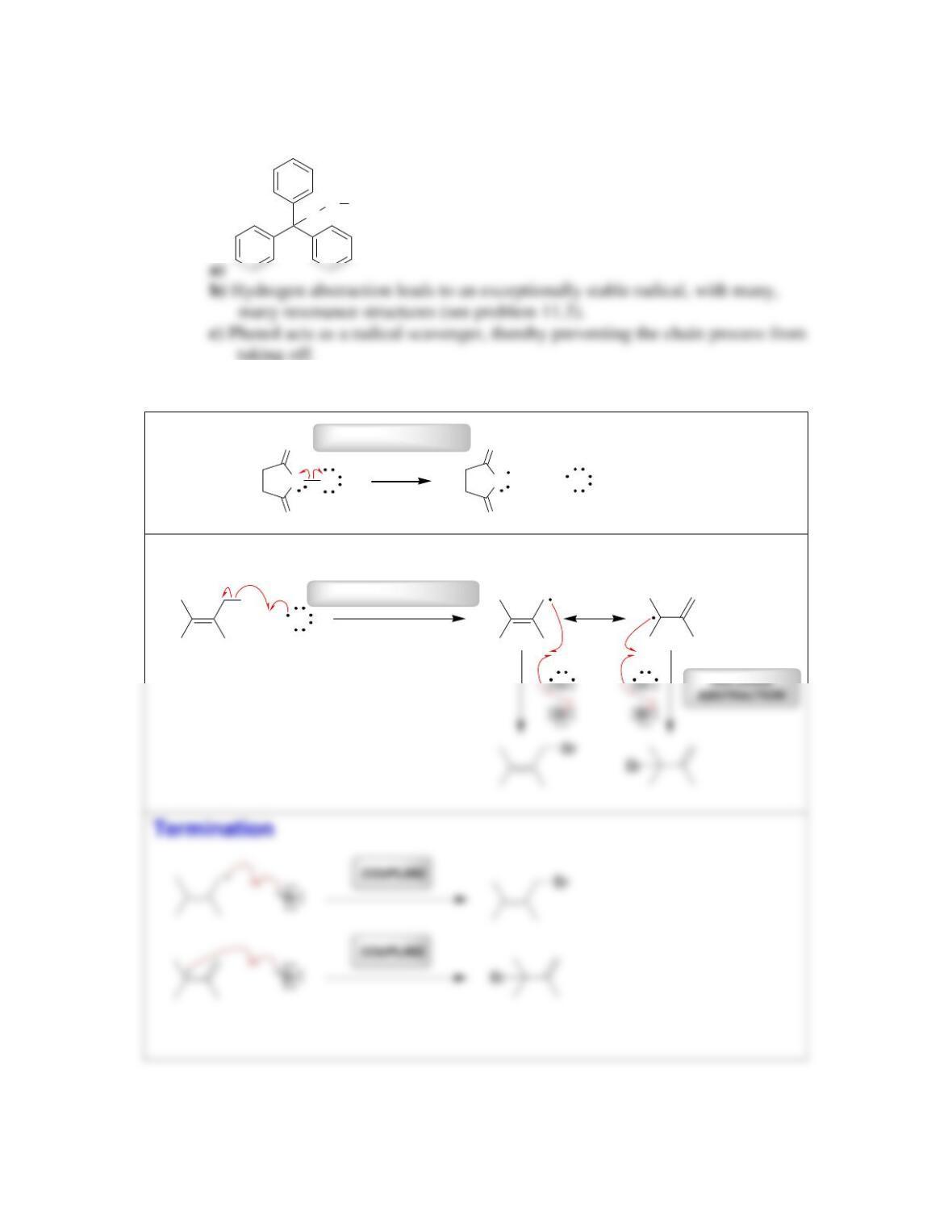
• Radical mechanisms utilize fishhook arrows, each of which represents the flow
of _____________________.
• Every step in a radical mechanism can be classified as initiation, ____________,
or termination.
• A radical initiator is a compound with a weak bond that readily undergoes
________________________.
an alkane.
Review of Skills
Fill in the blanks and empty boxes below. To verify that your answers are correct, look
in your textbook at the end of Chapter 11. The answers appear in the section entitled
SkillBuilder Review.
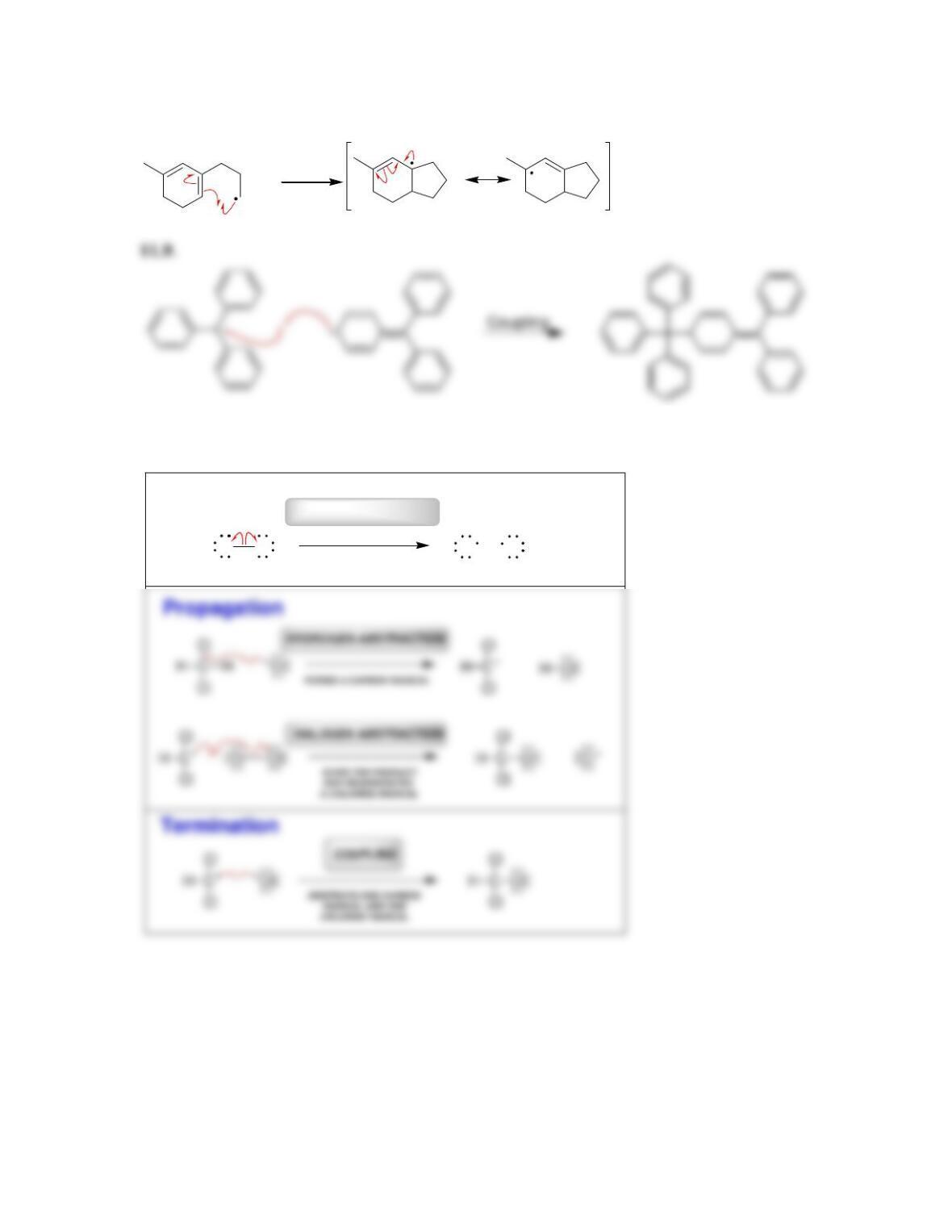
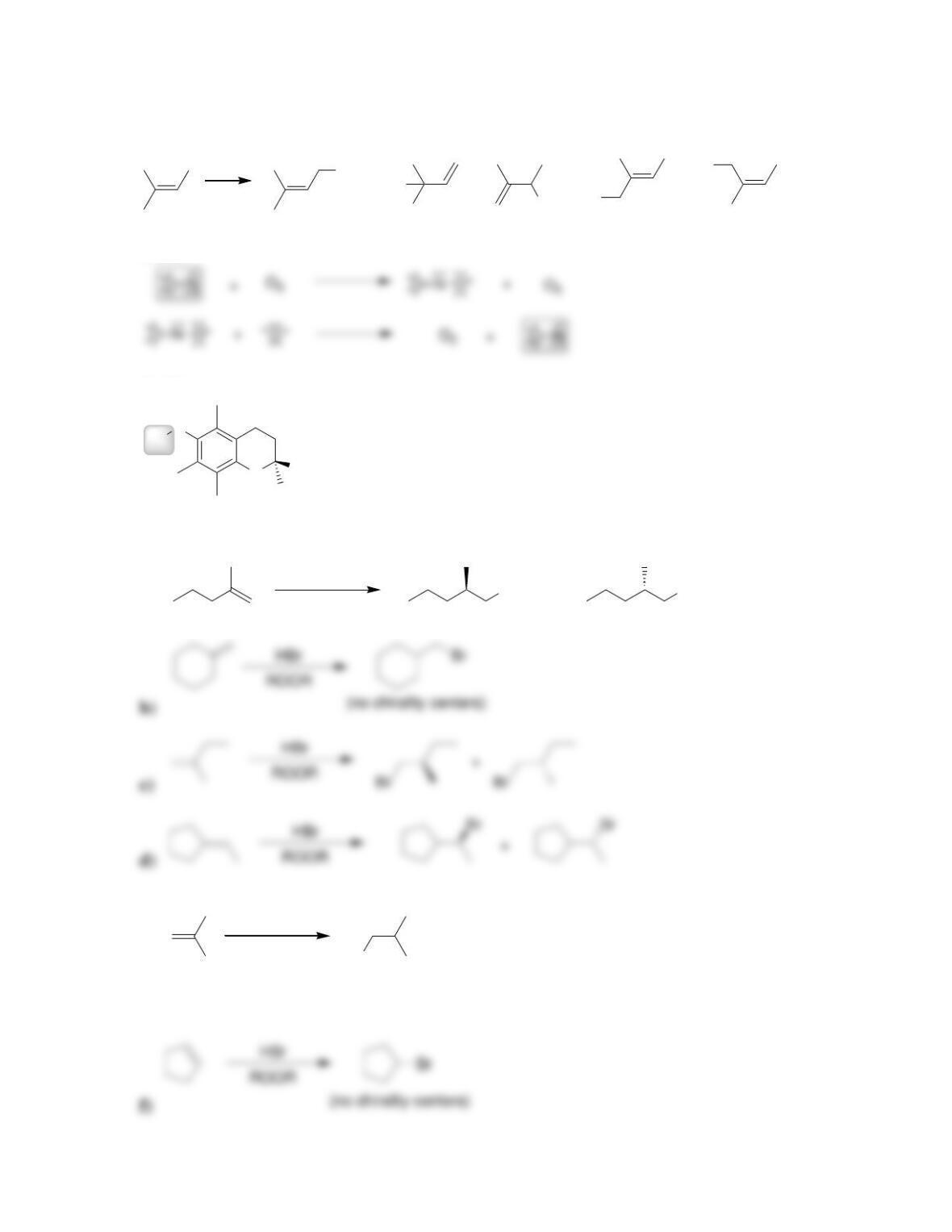
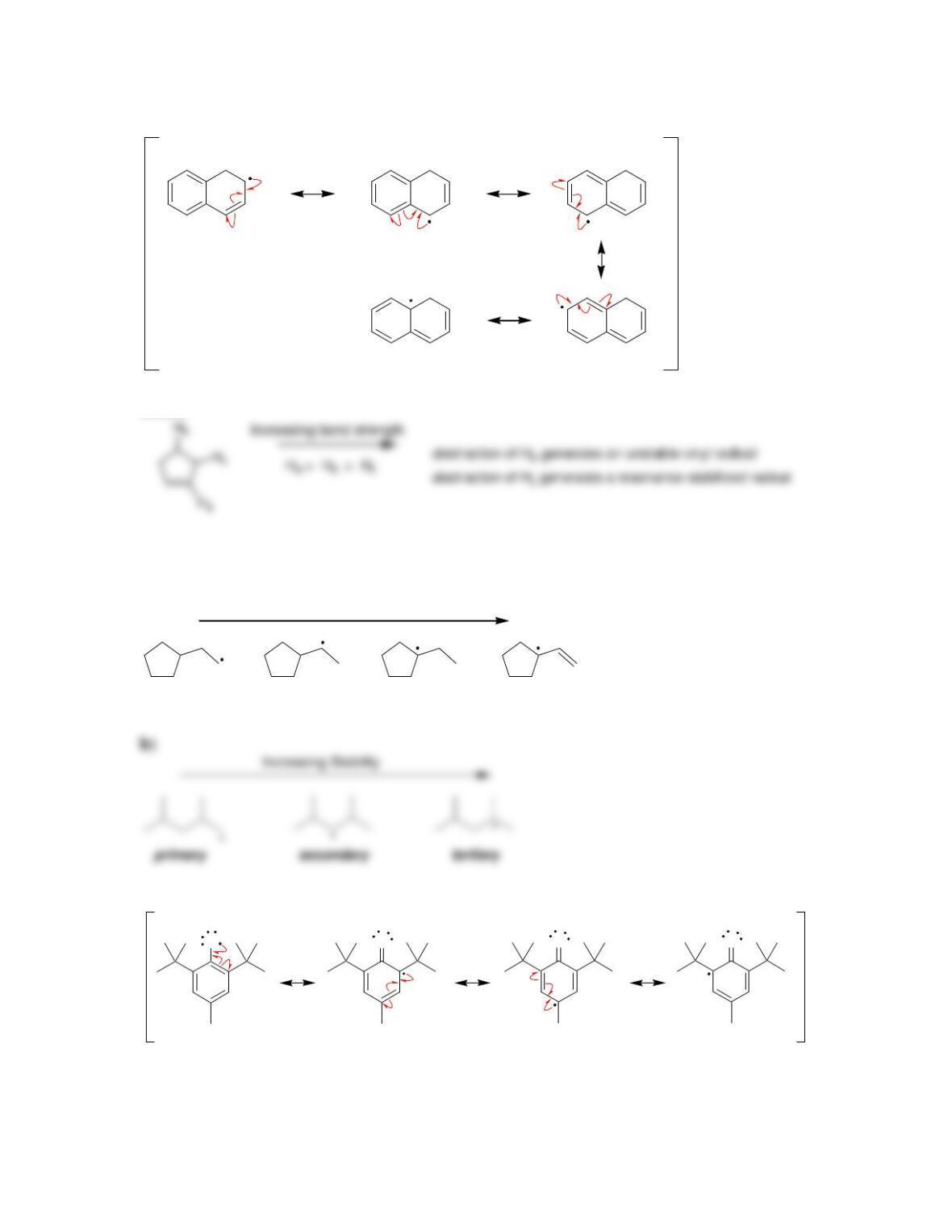
11.1 Drawing Resonance Structures of Radicals
DRAW A RESONANCE STRUCTURE OF THE RADICAL BELOW: